
 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A����ˮ����ʱ�����̶��ߣ��������������̶��� |
| B��û�н�ϴ��Һת������ƿ |
C������ƿϴ�Ӻ��ڱ���ˮ���δ������ ���� ���� |
| D������ʱ���ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������







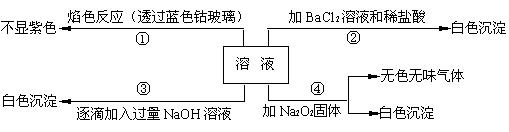 ��1����ԭ��Һ��һ�������ڵ������� ��
��1����ԭ��Һ��һ�������ڵ������� ��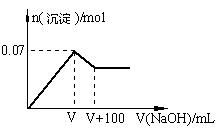
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����1���ȱ�����ֱ�Ӽ���AgNO3��Һ |
| B����1���ȱ�������ˮ��Ȼ�����AgNO3��Һ |
| C����1���ȱ����м���NaOH��Һ���������������ữ��Ȼ�����AgNO3��Һ |
| D����1���ȱ����м����Ҵ����������������ữ��Ȼ�����AgNO3��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ȥ��������������ϩ����������������ϼ��ȣ�����ϩת��Ϊ���� |
B����ȥ���е��������ӣ�����Ũ��ˮ�����ú���ˣ���ȥ���屽�ӳ��� |
| C����ȥ�������е������������������Һ�����ú��Һ |
| D���Ӻ�������ȡ�壺���ɺ������պ���ȴ����ˮ�����ˣ�����Һ����CCl4�����÷�Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ����Һ�������Լ�Ҳ�ܼ���������
����Һ�������Լ�Ҳ�ܼ���������| A���ۢ� | B���٢� | C���٢ڢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܢۢ٢ݢ� | B���ܢ٢ۢݢ� | C���٢ݢۢܢ� | D���ۢܢ٢ݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������ | B�����ȼ��� | C���� | D�����Ȼ�̼ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com