���𰸡�
����������Ԫ�����ڱ�֪��a-o��Ԫ�طֱ���H��Li��C��N��O��F��Na��Mg��Al��Si��S��Cl��Ar��K��Fe��
��1�������ڵ������� ��VIII�壮
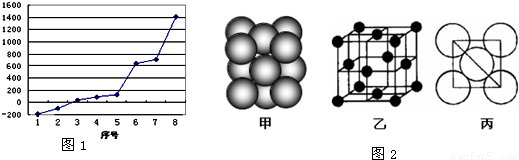
��2����������8��Ԫ�صĵ�����ֻ��SiΪԭ�Ӿ��壬�۷е�����۷е�Ϊԭ�Ӿ��壾�������壾���Ӿ��壬���Ӿ�����벡�������S���ף��ڷ��Ӿ�����Arֻ�з��Ӽ����������е���ͣ�������S������ֻ������Ϊ���壬���۷е�����������С��
���ݾ��������ж����ʵ��۷е�ߵͣ������۷е�ߵ�˳��Ϊ��ԭ�Ӿ��壾�������壾���Ӿ��壻�ǽ�����Խǿ��Ԫ����縺��Խ��
��3��ԭ�Ӿ����У�����Խ�̣���ѧ��Խǿ�����ʵ��۵�Խ�ߣ�
��4��k��l�γɵĻ�����kl
2�ǹ��ۻ����ԭ��֮���γɹ��ۼ������ݹ��ۻ��������ʽ����д������д�������³�Һ̬�������γɵľ���Ϊ���Ӿ��壮
��5�����þ�̯�����㾧���к��е�ԭ�Ӹ������ȸ�����֪��ԭ�Ӱ뾶���㾧������������ݸ�ԭ�ӵ����ԭ���������㾧���к���ԭ�ӵ��������ٸ����ܶȹ�ʽ�������ܶȣ�
��6��a��d���ɵ���������笠����ӣ�i���������������ӣ�a��d���ɵ������Ӻ�i�������ӿ���������γ�һ�ָ��Σ�����ε�Ũ��Һ����μ���Ũ����������Һ�������������Т���Һ�г��ְ�ɫ�����������д̼�����ζ����ų���˵���������������Ͱ������ڳ����������������ֱ�����ճ�����������˵������ƫ�����κ����ᱵ��
����⣺����Ԫ�����ڱ�֪��a-o��Ԫ�طֱ���H��Li��C��N��O��F��Na��Mg��Al��Si��S��Cl��Ar��K��Fe��
��1��ͨ�����Ϸ���֪��O��FeԪ�أ������ڱ��д��ڵ������ڡ���VIII�壮
�ʴ�Ϊ��FeԪ�أ��������ڡ���VIII�壮
��2����������8��Ԫ�صĵ�����ֻ��SiΪԭ�Ӿ��壬�۷е������ͼ��֪��š�8��������ΪSi�����Ӿ���ķе�ϵͣ��ǽ�����Խǿ�ĵ縺��Խ���Ӿ�����벡�������S���ף��ڷ��Ӿ�����Arֻ�з��Ӽ����������е���ͣ�������S������ֻ������Ϊ���壬���۷е�����������С����ͼ��֪����š�2��������ΪCl����縺�����
�ʴ�Ϊ��Si��2��
��3����jԭ�Ӹ�cԭ����1��1������϶��γɵľ���ΪSiC��SiC�����뾧��Si��ͬ������ԭ�Ӿ��壬����C��ԭ�Ӱ뾶С��SiC��C-Si�������Ⱦ���Si��Si-Si�����̣�C-Si���ܴ����SiC�����۷е�ߣ�
�ʴ�Ϊ��Si-C����SiC�����뾧��Si����ԭ�Ӿ��壬����C��ԭ�Ӱ뾶С��SiC��C-Si�������Ⱦ���Si��Si-Si�����̣����ܴ�����۷е�ߣ�
��4��k��l�γɵĻ�����kl
2��SCl
2����ԭ��һ��ԭ��֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ
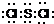
��SCl
2�����³�Һ̬��SCl
2���ڷ��Ӿ��壮
�ʴ�Ϊ��
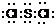
�����Ӿ��壮
��5���ɾ����ṹ��֪��������iԭ�ӵ���ĿΪ8×

+6×

=4������Ϣ��֪����ͼ��֪Ϊ�������������ⳤΪx������2x
2=��4d��
2������x=2
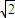
d������������ⳤΪ2
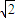
d������������Ϊ16
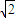
d
3���þ�����ԭ�ӵ�����=4
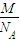
���������ܶ�=
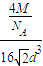
=
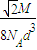
��
�ʴ�Ϊ��4��
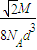
��
��6��a��d���ɵ���������笠����ӣ�i���������������ӣ�a��d���ɵ������Ӻ�i�������ӿ���������γ�һ�ָ��Σ�����ε�Ũ��Һ����μ���Ũ����������Һ�������������Т���Һ�г��ְ�ɫ�����������д̼�����ζ����ų���˵�������������������ᱵ�Ͱ������ڳ����������������ֱ�����ճ�����������˵����������������������Ӧ����ƫ�����Σ��������ӷ���ʽΪ��NH
4++Al
3++5OH
-+2SO
42-+2Ba
2+=NH
3��+3H
2O+AlO
2-+2BaSO
4����
�ʴ�Ϊ��NH
4++Al
3++5OH
-+2SO
42-+2Ba
2+=NH
3��+3H
2O+AlO
2-+2BaSO
4����
���������⿼���˾��������뵥���۷е�Ĺ�ϵ��������ܶȵ�֪ʶ�㣬�ѵ��Ǽ��㾧����ܶȣ�����ȷȷ�������к��е�ԭ�Ӹ���������������ǽ�����Ĺؼ�����ȷ��ͬ���͵ľ������۷е�ߵ͵��жϷ�����

 ��SCl2�����³�Һ̬��SCl2���ڷ��Ӿ��壮
��SCl2�����³�Һ̬��SCl2���ڷ��Ӿ��壮 �����Ӿ��壮
�����Ӿ��壮 +6×
+6× =4������Ϣ��֪����ͼ��֪Ϊ�������������ⳤΪx������2x2=��4d��2������x=2
=4������Ϣ��֪����ͼ��֪Ϊ�������������ⳤΪx������2x2=��4d��2������x=2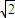 d������������ⳤΪ2
d������������ⳤΪ2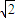 d������������Ϊ16
d������������Ϊ16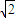 d3���þ�����ԭ�ӵ�����=4
d3���þ�����ԭ�ӵ�����=4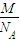 ���������ܶ�=
���������ܶ�=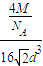 =
=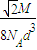 ��
��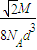 ��
��

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�





 NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ��
NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ�� ��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����
��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����