
| A£®¢Ł | B£®¢Ś¢Ü | C£®¢Ū¢Ü | D£®¢Ł¢Ś¢Ū |
 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 O2(g)=H2O(l) ”÷H=£285.84kJ”¤mol£1
O2(g)=H2O(l) ”÷H=£285.84kJ”¤mol£1 ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 2SO3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
2SO3(g)·“Ó¦¹ż³ĢµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ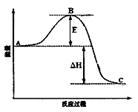
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 O2(g)==H2O(l) ”÷H3= £285 kJ”¤mol-1
O2(g)==H2O(l) ”÷H3= £285 kJ”¤mol-1| A£®”Ŗ243.5 kJ”¤mol-1 | B£®”Ŗ487 kJ”¤mol-1 | C£®”Ŗ1113.5 kJ”¤mol-1 | D£®”Ŗ2227 kJ”¤mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
 H="-24.8" kJ”¤mol-1
H="-24.8" kJ”¤mol-1 H="-47.2" kJ”¤mol-1
H="-47.2" kJ”¤mol-1 H="+640.5" kJ”¤mol-1
H="+640.5" kJ”¤mol-1| A£®-218 kJ”¤mol-1 | B£®-109kJ”¤mol-1 | C£®+218 kJ”¤mol-1 | D£®+109 kJ”¤mol-1 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
 CH3OH(g) ¦¤H1
CH3OH(g) ¦¤H1 CH3OH(g) + H2O(g) ¦¤H2
CH3OH(g) + H2O(g) ¦¤H2

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

| A£®N4ŹōÓŚŅ»ÖÖŠĀŠĶµÄ»ÆŗĻĪļ | B£®N4ŹĒN2µÄĶ¬ĻµĪļ |
| C£®N4×Ŗ±äĪŖN2ŹĒĪļĄķ±ä»Æ | D£®1molN4ĘųĢå×Ŗ±äĪŖN2·Å³ö724kJÄÜĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ÓŠ10NAøöµē×Ó×ŖŅĘŹ±£¬øĆ·“Ó¦ĪüŹÕ1300kJµÄÄÜĮæ |
| B£®ÓŠNAøöĖ®·Ö×ÓÉś³ÉĒŅĪŖŅŗĢ¬Ź±£¬ĪüŹÕ1300kJµÄÄÜĮæ |
| C£®ÓŠNAøöĢ¼Ńõ¹²ÓƵē×Ó¶ŌŠĪ³ÉŹ±£¬·Å³ö1300kJµÄÄÜĮæ |
| D£®ÓŠ8NAøöĢ¼Ńõ¹²ÓƵē×Ó¶ŌŠĪ³ÉŹ±£¬·Å³ö1300kJµÄÄÜĮæ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®H2O ( g ) £½ H2 ( g ) + 1/2O2 ( g )”÷H =" +242" kJ/mol |
| B£®2H2 ( g ) + O2 ( g ) = 2H2O ( l )”÷H = £484 kJ/mol |
| C£®H2 ( g ) + 1/2O2 ( g ) = H2O ( g )”÷H =" +242" kJ/mo |
| D£®2H2 ( g ) + O2 ( g ) = 2H2O ( g )”÷H =" +484" kJ/mol |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com