
�����£���20 mL����������Һ����μ���0.2 mol��L��1��CH3COOH��Һ���ζ�������ͼ��ʾ��
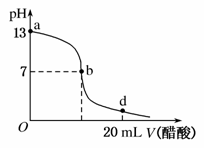
(1)������������Һ�����ʵ���Ũ��Ϊ______________��
(2)��b�㣬c(Na��)____ ____c(CH3COO��)(�����������������)��
(3)����������Һ��CH3COOH��Һǡ����ȫ��Ӧ�ĵ�λ�����ߵ�____ ____(��ѡ��ı��)��
A��a�� B��b�� C��d�� D��a��b���ij�� E��b��d���ij��
(4)��d�㣬��Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ
____________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��״���£���a LSO2��Cl2��ɵĻ������ͨ��100mL0.1mol��L-1Fe2(SO4)3��Һ�У���ַ�Ӧ����Һ���ػ�ɫ��dz����Ӧ�����Һ�м���������BaCl2��Һ�������ó������ˡ�ϴ�ӡ��������أ�������Ϊ11.65g�������й��ڸù��̵��ƶϲ���ȷ����
A�����������SO2�����Ϊ0.448L(���) B�����ó���Ϊ0.05mol��BaSO4
C��a L�����������ʵ���Ϊ0.04mol D��a��ȡֵ��ΧΪ 0.672��a��0.896
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��2KMnO4��16HCl 2KCl��2MnCl2��5Cl2����8H2O��Ӧ�У�����������
2KCl��2MnCl2��5Cl2����8H2O��Ӧ�У�����������
��A��KCl�������� B��MnCl2�������� C��H2O ��������D��Cl2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾˮ��c(H��)��c(OH��)�Ĺ�ϵ�������жϴ������(����)
A������������������c(H��)��c(OH��)��KW
B��M��������������c(H��)��c(OH��)
C��ͼ��T1��T2
D��XZ������������pH��7
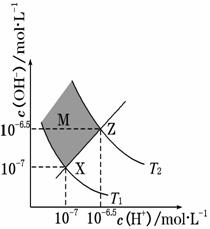
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���0.100 0 mol��L��1 NaOH��Һ�ζ�20.00 mL 0.100 0 mol��L��1 CH3COOH��Һ�����õζ�������ͼ��ʾ������˵����ȷ����(����)
A�������ʾ��Һ�У�c(CH3COO��)��c(OH��)��c(CH3COOH)��c(H��)
B�������ʾ��Һ�У�c(Na��)��c(CH3COOH)��c(CH3COO��)
C�������ʾ��Һ�У�c(Na��)��c(OH��)��c(CH3COO��)��c(H��)
D���ζ������п��ܳ��֣�
c(CH3COOH)��c(CH3COO��)��c(H��)��c(Na��)��c(OH��)
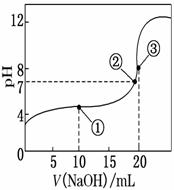
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л������������������ͬ��ͬ���칹����Ŀ(�����������칹)������
A������ B���촼
C����ϩ D���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ǵؿ��к�����ߵĽ���Ԫ�أ��䵥�ʡ��Ͻ��仯���������������е�Ӧ�������㷺�������������������仯�������Ҫԭ�ϡ�
��1����Ԫ����Ԫ�����ڱ��е�λ���� ��
��2�������������Խ����һ����������价������ȫ���ܵ�Խ��Խ��Ĺ�ע����ԭ����
ͼ��ʾ��
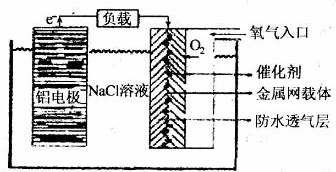
�ٸõ�ص��ܷ�Ӧ��ѧ����ʽΪ ��
�ڵ����NaCl�������� ��
������һ�������Ϊ��Դ���KI��Һ��ȡKIO3��ʯīΪ�缫���ϣ�ʱ���������������ĵ缫��ӦʽΪ ��
��ij��һ������ص�Ч��Ϊ50%������������Դ���500mL�ı���NaCl��Һ����������������Һ��������Һ���ǰ��������䣩��NaOH��Ũ��Ϊ0.3 mol��L��1����ù�����������������Ϊ ��
��3���Ȼ����㷺�����л��ϳɺ�ʯ��ҵ�Ĵ��������Ȼ���Ҳ�����ڳ�����ˮ������
���Ȼ����ڼ�������������������̬�Ȼ����Ļ�ѧʽΪAl2Cl6��ÿ��Ԫ�ص�ԭ���������ﵽ8�����ȶ��ṹ������ṹʽΪ ��
�ڽ����������̼�ۻ�Ϻ���Ȳ�ͨ���������ɵõ��Ȼ�����ͬʱ����CO��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��Ӳ֬���������NaOH��Һ���Ƚ���������Ӧ���ܰ�Ӳ֬���ƺ��ʹӻ�����з�������IJ����У����������ڹ��ˣ������ܷ�Һ��������ȷ������ǣ� ��
A���٢ڢ� B���٢ۢ� C���ڢۢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪������п��صĵ缫��Ӧ��пƬ��Zn��2OH����2e�� �� ZnO �� H2O��
ʯī��1/2 O2��H2O ��2e�� �� 2OH��,�ݴ��ƶ�пƬ��
A.������������ B.����������ԭ C.������������ D.����������ԭ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com