
A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬C
Ϊ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ(����ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ)��
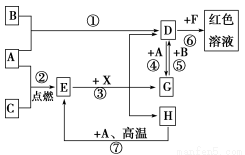
(1)д����ѧʽ��A________��D________��E________��X________��
(2)�ڷ�Ӧ�������У�������������ԭ��Ӧ����________(����)��
(3)��Ӧ�������ӷ���ʽΪ��_______________________________________��
(4)��Ӧ���Ļ�ѧ����ʽΪ_________________________________________��
�÷�Ӧ��ÿ����0.3 mol��A����ת�Ƶ���______mol��
(5)д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽ��_________________________
(1)Fe��FeBr3��Fe3O4��HBr��(2)�ۢ�
(3)Fe3����3SCN��=Fe(SCN)3
(4)3Fe��4H2O(g) Fe3O4��4H2��0.8
Fe3O4��4H2��0.8
(5)Fe3����3HCO3��=Fe(OH)3����3CO2��
�������������ϢA��B��CΪ��ѧ�������ʣ�����һ��Ϊ������BΪҺ�壬������ȷ��BΪBr2��AΪ���壬��ȷ��AΪ����������D��G��ת����ϵ���Ʋ�A���ڱ�۽���Ԫ�أ���һ��ȷ��AΪFe��EΪ��ɫ���壬����һ������ǿ�ᷴӦ�������������ʣ��²�EΪFe3O4��HΪH2O��DΪ FeBr3��GΪFeBr2��XΪHBr��FΪ���軯�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��4-4��ϰ���������棩 ���ͣ�ʵ����
ij��ѧ����С����ʵ�����������ͼ��ʾ��ʵ��װ�ã����������Ĵ�������ʵ�顣
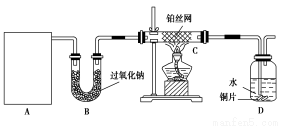
(1)A�������巢��װ�ã�A�����õ��Լ�ֻ�ܴ�����������ѡȡ��
������泥���̼��泥���̼����泥����Ȼ�泥�����ʯ�ң����������ơ�
��A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ������ (��ѡ����)����ֻ��һ��ҩƷ��ȡ����ʱ��ͼ�пհ״���������ӦΪ (ѡ������������ţ��̶�װ��ʡ��)��

(2)��װ�ò�����������Ȼ����һ����ȱ�ݣ��ԴӰ�ȫ�뻷���ĽǶ������ǣ��Ը�װ�ý��иĽ���
�� ��
�� ��
(3)���ոĽ����װ�ý���ʵ�飬������������⣺
��װ��B�������� ��
��д��C�з�����Ӧ�Ļ�ѧ����ʽ�� ��
����A��B���Լ���������װ��D�п��Թ۲쵽��ʵ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��4-1��ϰ���������棩 ���ͣ�ʵ����
Ϊ��֤��һ����̼���л�ԭ�ԣ��������������ʵ�飺
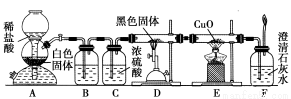
(1)װ��B�������˵��Լ��� ��
(2)װ��D�з�����Ӧ�Ļ�ѧ����ʽ�� �� ��
(3)������װ��C���ճ�ȥ������ˮ������������ �� ��
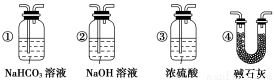
(4)������F��ʯ��ˮ����ǵ�����Ҳ��ȷ��CO���л�ԭ�ԣ�Ӧ����ͼ��װ�� �� ֮����������װ���е� (�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��3-4��ϰ���������棩 ���ͣ�ѡ����
�����£�����H2SO4��CuSO4��Һ����μ��뺬a mol
���ʵ�NaOH��Һ��ǡ��ʹ��Һ��pH��7�����������������(����)
A����Ӧ����Һ��c(Na��)��2c(SO42��)
B��a/2 mol�����������ʵ�����0
C������������Ϊ49 a g
D����Һ��n(SO42��)��a/2 mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��3-4��ϰ���������棩 ���ͣ�ѡ����
���й��������ֵ���������ȷ����(����)
A��������ʯ�������ǻ�ѧ�仯�����������ɸ��������仯
B������������ʢ������ͭ��Һ�������ױ���ʴ
C�������ֶ�������̼�Ͻ����������ܺ���;�ϲ��
D�����Ƕ�����ȫ�ܽ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��3-3��ϰ���������棩 ���ͣ�ѡ����
A��B��C����ѧ�������ֵ��ʣ�A��B��C����������֮��ķ�Ӧ��ϵ��ͼ��ʾ������B��D��E��F��ˮ��Һ�����ԡ���D��C�D��E��F�ҳ�����ֻ��BΪ��̬��
��A��B��C�ֱ�Ϊ(����)
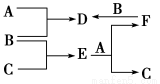
A��Fe��Cl2��Cu B��Fe��Cl2��H2
C��Cu��Cl2��Fe D��Fe��Cl2��Mg
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��3-2��ϰ���������棩 ���ͣ������
��0.1 mol��þ�������������100 mL 2 mol/L H2SO4��Һ�У�Ȼ���ٵμ�1 mol/L NaOH��Һ����ش�
(1)���ڵμ�NaOH��Һ�Ĺ����У���������m�����NaOH��Һ�����V�仯����ͼ��ʾ����V1��160 mLʱ���������ĩ��n(Mg)��________mol��V2��______________mL��
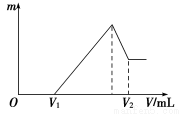
(2)���ڵμ�NaOH��Һ�����У���ʹMg2����Al3���պó�����ȫ�������NaOH��Һ�����V(NaOH)��____________mL��
(3)���������Ϊ0.1 mol������þ�۵����ʵ�������Ϊa����100 mL 2 mol/L�������ܽ�˻������ټ���450 mL 1 mol/L��NaOH��Һ�����ó�������Al(OH)3�������������a��ȡֵ��Χ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��3-1��ϰ���������棩 ���ͣ������
��A��B���ֳ�����������ɵĻ�������ɫ��Ӧ��Ϊ��ɫ�����ת����ϵ��ͼ(��
�����ʾ���ȥ)��

�������Ϲ�ϵ���ش��������⣺
(1)д��A��B��C��D�Ļ�ѧʽ��A________��B________��C________��D________��
(2)д��������м�ˮ��Ӧ�Ļ�ѧ����ʽ��_____________________________________
(3)���Ⱥ���ֻ�õ�һ�ֹ��廯�����A��B�����ʵ���֮�ȵ����ֵΪ________(������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��߿���ѧһ�ָ�ϰ�κ�淶ѵ��2-1��ϰ���������棩 ���ͣ������
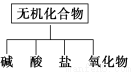
��������ɸ�������ɺ����ʽ��з��ࣺ
(1)��ͼ��ʾ�����ʷ����������________
(2)��Na��K��H��O��C��S��N�������ֻ�����Ԫ����ɺ��ʵ����ʣ��ֱ������±��ڢۢ��ĺ��档
������� | �� | �� | �� | ������ |
��ѧʽ | HCl��____ | ��___��Ba(OH)2 | ��Na2CO3��____ | ��CO2��Na2O2 |
(3)д����ת��Ϊ���Ļ�ѧ����ʽ_____________________________
(4)���������������ΪO2��Դ�ķ�Ӧԭ��Ϊ��___________________________
(5)ʵ�����Ʊ�������________��________��Ӧ�����������ķ�����____________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com