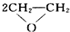ЁАЪЎЖўЮхЁБЙцЛЎжаЕФЛЗБЃШЮЮёЪЧЕН2015ФъФЉЃЌЮвЙњЕФжївЊЮлШОЮяЖўбѕЛЏЬМБШ2010ФъЯћМѕ10%ЃЎФГЛЏбЇаЫШЄаЁзщЩшМЦСЫгУЭКЭХЈСђЫсжЦШЁSO
2ЃЌВЂвРДЮНјааМьбщSO
2ОпгаЫсадбѕЛЏЮяЕФаджЪЁЂЛЙдадЁЂЦЏАзадКЭбѕЛЏадЕФЪЕбщЃЌЫљгУвЧЦїШчЭМЫљЪОЃЈЭМжаСЌНгНКЙмЁЂМаГжКЭМгШШзАжУОљвбЪЁТдЃЉЃК
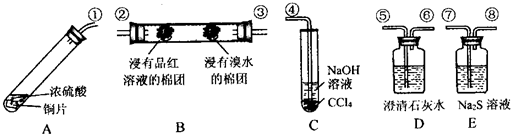
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЩЯЪізАжУЕФНгПкСЌНгЫГађвРДЮЪЧЂйЁњ
ЂнЁњЂоЁњЂлЁњЂкЁњЂпЁњЂрЁњЂм
ЂнЁњЂоЁњЂлЁњЂкЁњЂпЁњЂрЁњЂм
ЃЈЬюЕМЙмБрКХЃЉЃЎ
ЃЈ2ЃЉзАжУAжаЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЪЧ
Cu+2H
2SO
4ЃЈХЈЃЉ
CuSO
4+2SO
2Ёќ+2H
2O
Cu+2H
2SO
4ЃЈХЈЃЉ
CuSO
4+2SO
2Ёќ+2H
2O
ЃЎ
ЃЈ3ЃЉжЄУїSO
2ОпгабѕЛЏадЕФЯжЯѓЪЧ
EЪдЙмжаШмвКБфЛызЧ
EЪдЙмжаШмвКБфЛызЧ
ЃЛжЄУїSO
2ОпгаЛЙдадЕФЪЕбщжаЃЌЗДгІЕФРызгЗНГЬЪНЪЧ
SO2+Br2+2H2O=4H++SO42-+2Br-
SO2+Br2+2H2O=4H++SO42-+2Br-
ЃЎ
ЃЈ4ЃЉCЪдЙмжаNaOHШмвКЕФзїгУЪЧ
ЮќЪеЮДЗДгІЕФЖўбѕЛЏСђЃЌвдУтЮлШОПеЦј
ЮќЪеЮДЗДгІЕФЖўбѕЛЏСђЃЌвдУтЮлШОПеЦј
ЃЌCCl
4ЕФзїгУЪЧ
ЗРжЙЕЙЮќ
ЗРжЙЕЙЮќ
ЃЎ
ЃЈ5ЃЉФГЙЄГЇгУЪЏЛвЪЏ--ЪЏИрЪЊЗЈбЬЦјЭбСђЙЄвеРДНЕЕЭКЌСђШМСЯШМЩеХХЗХЕФSO
2ЃЌЭЌЪБЛёЕУЪьЪЏИрЃЈ2CaSO
4?H
2OЃЉЃЌБЃЛЄСЫЛЗОГЃЎ
ЂйИУЙЄвеРћгУСЫSO
2ЕФЪВУДаджЪЃП
ЫсадЁЂЛЙдад
ЫсадЁЂЛЙдад
ЃЎ
ЂкаДГіИУЙЄвеЕФЛЏбЇЗНГЬЪН
2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2
2CaCO3+2SO2+O2+H2O=2CaSO4?H2O+2CO2
ЃЎ




 УћаЃПЮЬУЯЕСаД№АИ
УћаЃПЮЬУЯЕСаД№АИ