
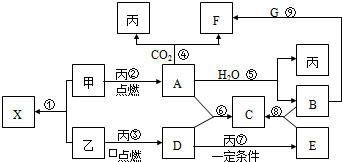
��10�֣��ס��ҡ���Ϊ�������ʣ��ҡ�����Ԫ�������ڱ���λ��ͬһ���塣X��A��B��C��D��E��F��G��Ϊ�����Ļ��������A��X��Ħ��������ͬ��A��G����ɫ��ӦΪ��ɫ����һ�������£��������ת����ϵ����ͼ��
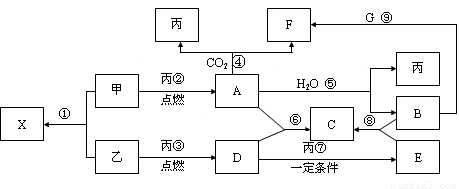
��ش�
��1�� д��ѧʽ����_________��E________��
��2����X�ĵ���ʽΪ____________________��
��3��д��A��H2O��Ӧ�Ļ�ѧ��Ӧ����ʽ��__________________ _____________����Ӧ����������__________��1molA�μӷ�Ӧת�Ƶ���_____mol��
��4��д����Ӧ������ӷ���ʽ��___________ _____________��
��5������F��������__________��_________������Һ��_____�ԣ�ԭ����_______________________________����һ�����ӷ���ʽ��ʾ��
����14�֣���1��O2 �� 1�֣� SO3 �� 1�֣�
��2�� �� 1�֣�
�� 1�֣�
��3��2 Na2O2��2 H2O == 4 NaOH ��O2�� �� 2�֣� Na2O2 �� 1�֣� 1 �� 1�֣�
��4�� HCO3-+H+ = CO32-+ H2O �� 2�֣�
��5������ �� 1�֣� �մ� �� 1�֣� �� �� 1�֣�
CO32-+ H2O  HCO3-+ OH- �� 2�֣�
HCO3-+ OH- �� 2�֣�
������������������ͼ�⣬�ؼ�����ͻ�Ƶ㡣A��G����ɫ��ӦΪ��ɫ����A�ܺ�CO2��ˮ����Ӧ���ɱ�������A�ǹ������ƣ�B���������ƣ�F��̼���ƣ��������������ݷ�Ӧ���֪��GӦ����̼�����ơ����ݷ�Ӧ�ڿ�֪�������ơ�����Ϊ�ҡ�����Ԫ�������ڱ���λ��ͬһ���壬��������S����X�����ƣ�D��SO2��E����������SO2���л�ԭ�ԣ��ܱ����������������������ƣ�����C�������ơ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
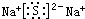
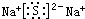
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
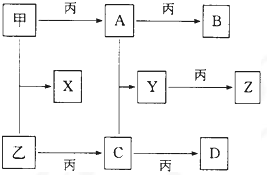
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com