
 �������� �����������μ�ˮ��ʹ��Һ�İ�Һ�����ø��̶������У�������ƿ����ҡ�ȡ�
�������� �����������μ�ˮ��ʹ��Һ�İ�Һ�����ø��̶������У�������ƿ����ҡ�ȡ� ,ƫ�ͻ��Dz���?
,ƫ�ͻ��Dz���? | A����ˮʱԽ���̶���______ | B�����ǽ�ϴ��Һ��������ƿ______ |
C������ƿ�ڱڸ���ˮ���δ���ﴦ�� | D���ܽ��û����ȴ����ж��� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Ϊ�˼ӿ�������ʣ����ò��������Ͻ���������е�Һ�� |
| B�����ü���ʼ���KI��Һ�͵�����Һ |
| C������pH��ֽ�ⶨ���Ƶ���ˮ��pH |
| D�����ò�����պȡ�����������ʵ�Ũ��Һ����ɫ��Ӧʵ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������A13+��NH4+��Cl- | B���϶���A13+��Ba2+��HCO3- |
| C������û��K+��HCO3-��NH4+ | D���϶�û��A13+��SO32-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 ɫ���____________ɫ��
ɫ���____________ɫ��| �ζ����� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na2CO3��Ba(NO3)2��HNO3 | B��BaCl2��Na2CO3��HCl |
| C��Ba(NO3)2��Na2CO3��HNO3 | D��Ba(NO3)2��K2CO3��HNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
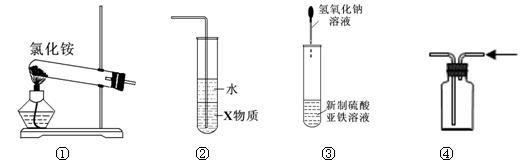
| A��ʵ������װ�â���ȡ���� |
| B��װ�â���X��Ϊ���Ȼ�̼�����������հ���������ֹ���� |
| C��װ�âۿ�����ʵ�����ռ�HCl��Cl2 |
| D��װ�âܿ������Ʊ��������������۲�����ɫ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com