
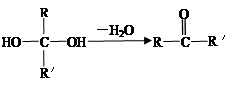
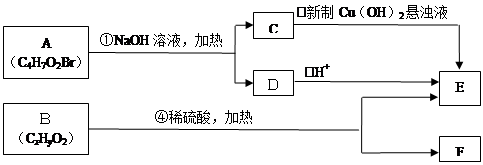
 CH3COOH+Cu2O��+2H2O
CH3COOH+Cu2O��+2H2O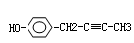
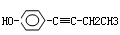

 ����4�֣�
����4�֣� CH3COOH+Cu2O��+2H2O
CH3COOH+Cu2O��+2H2O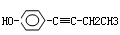
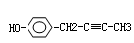 ����Fȡ�����к���2��̼̼˫����2��̼̼˫����������һ����F�Ľṹ��ʽΪ
����Fȡ�����к���2��̼̼˫����2��̼̼˫����������һ����F�Ľṹ��ʽΪ



 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ͬ�����£������顢�����顢������ķе��������� |
| B������ױ���Ϊͬϵ�����ʹKMnO4������Һ��ɫ |
| C���ױ���Cl2�����µķ�Ӧ���Ҵ�������ķ�Ӧ����ͬһ���͵ķ�Ӧ |
| D���з�����ζ��C9H18O2�����������¼��ȿ�ˮ�������Է���������ͬ�������л������ϴ�������C9H18O2�Ľṹ��16�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
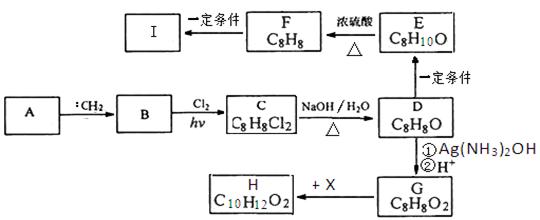
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��9�� | B��12�� | C��15�� | D��18�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CnH2n��6(n��11) | B��CnH2n��8(n>10) | C��CnH2n��10(n��10) | D��CnH2n��12(n��10) |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
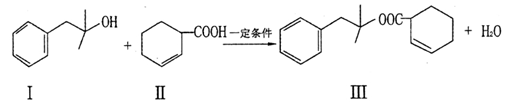

 Ҳ�ܷ�������V
Ҳ�ܷ�������V ���ķ�Ӧ����д�������ɴ��Ľṹ��ʽ ��
���ķ�Ӧ����д�������ɴ��Ľṹ��ʽ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
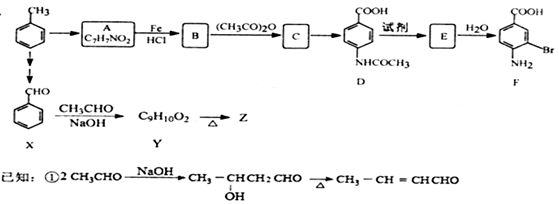
 �����а����ױ�������
�����а����ױ��������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com