
ʵ���⣺(18�֣�ÿ��2��)
�֣�
�ס��ҡ�����λͬѧ�ֱ�������ʵ��װ�ü���ѧҩƷ����ʯ��Ϊ�������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش����⣺

��1��������ȡ�����Ļ�ѧ����ʽΪ�� ��
��2����λͬѧ���������ſ������ռ���������ԭ���� ��
��3����λͬѧ������װ����ȡ����ʱ,������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___��(���ס������ҡ�����)��
��4�����鰱���Ƿ��ռ����ķ�����:��������������������ͽ��ۣ�
��5����λͬѧ����Ϊ���ǵ�ʵ��װ�û������ڼ���̼����粒�������ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���________(��ס������ҡ�����)����װ���е�NH4HCO3�����ܷ���NH4Cl������棿____ (��ܡ����ܡ�)��
��
��֪���������ʹ���������Һ��ɫ��Ӧ�Ļ�ѧ����ʽΪ��
5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4
��ͼΪ��֤Ũ������ľ̿�ڼ��������£���Ӧ�������Ƿ���SO2��CO2�IJ���װ�á�

��6��ʵ��ʱ���� ������ʢ�г���ʯ��ˮ��ʵ��װ��(��a��b ���)��
��7���ɹ۲쵽Aƿ��Һ ��
��8��Cƿ��Һ�������� ��
1��2NH4Cl+Ca��OH��2��CaCl2+2NH3 ��+2H2O��2�֣�
��2��NH3���ܶ�С�ڿ�����2�֣�
��3���ң�2�֣�
��4����ʪ��ĺ�ɫʯ����ֽ���ڹܿڣ�����ֽ��������˵�����ռ���������պ��Ũ����IJ����������Թܿڣ����а��̲�������֤�����ռ�������2�� ���в����������۸�1�֣�
��5������ ���ܣ�4�� ��2�֣�
��6��b ��7����ɫ ��8������SO2�Ƿ������
����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���⣺(18�֣�ÿ��2��)
�֣�
�ס��ҡ�����λͬѧ�ֱ�������ʵ��װ�ü���ѧҩƷ����ʯ��Ϊ�������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش����⣺
��1��������ȡ�����Ļ�ѧ����ʽΪ�� ��
��2����λͬѧ���������ſ������ռ���������ԭ���� ��
��3����λͬѧ������װ����ȡ����ʱ,������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___��(���ס������ҡ�����)��
��4�����鰱���Ƿ��ռ����ķ�����:��������������������ͽ��ۣ�
��5����λͬѧ����Ϊ���ǵ�ʵ��װ�û������ڼ���̼����粒�������ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���________(��ס������ҡ�����)����װ���е�NH4HCO3�����ܷ���NH4Cl������棿____ (��ܡ����ܡ�)��
��
��֪���������ʹ���������Һ��ɫ��Ӧ�Ļ�ѧ����ʽΪ��
5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4
��ͼΪ��֤Ũ������ľ̿�ڼ��������£���Ӧ�������Ƿ���SO2��CO2�IJ���װ�á�
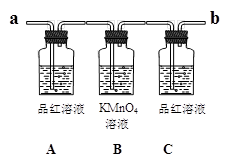
��6��ʵ��ʱ���� ������ʢ�г���ʯ��ˮ��ʵ��װ��(��a��b ���)��
��7���ɹ۲쵽Aƿ��Һ ��
��8��Cƿ��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ�������и�һ��ѧ����ĩ��ǰ���Ի�ѧ�Ծ� ���ͣ�ʵ����
ʵ���⣺(1 8�֣�ÿ��2��)
8�֣�ÿ��2��)
�֣�
�ס��ҡ�����λͬѧ�ֱ�������ʵ��װ�ü���ѧҩƷ����ʯ��Ϊ�������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش����⣺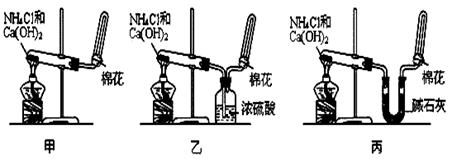
��1��������ȡ�����Ļ�ѧ����ʽΪ�� ��
��2����λͬѧ���������ſ������ռ���������ԭ���� ��
��3����λͬѧ�� ����װ����ȡ����ʱ,������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___��(���ס������ҡ�����)��
����װ����ȡ����ʱ,������һλͬѧû���ռ�������ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___��(���ס������ҡ�����)��
��4�����鰱���Ƿ��ռ����ķ�����:��������������������ͽ��ۣ�

��5����λͬѧ����Ϊ���ǵ�ʵ��װ�û������ڼ���̼����粒�������ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���________(��ס������ҡ�����)����װ���е�NH4HCO3�����ܷ���NH4Cl������棿____ (��ܡ����ܡ�)��
��
��֪���������ʹ���������Һ��ɫ��Ӧ�Ļ�ѧ����ʽΪ��
5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4
��ͼΪ��֤Ũ������ľ̿�ڼ��������£���Ӧ�������Ƿ���SO2��CO2�IJ���װ�á�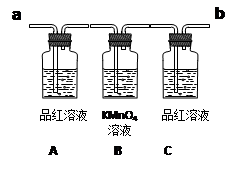
��6��ʵ��ʱ���� ������ʢ�г���ʯ��ˮ��ʵ��װ��(��a��b ���)��
��7���ɹ۲쵽Aƿ��Һ ��
��8��Cƿ��Һ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��ˮ�ж��и���ģ�⣨5�£����Ի�ѧ�Ծ����������� ���ͣ�ʵ����
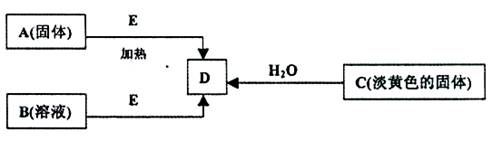
��ÿ��2�֣���18�֣�����A��B��C��D��E�������ʣ�����������ת����ϵ������EΪ��ɫ��ĩ������ͼת���о�������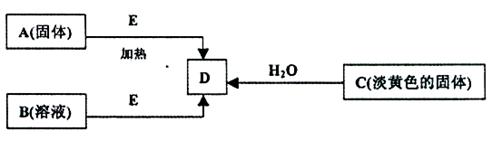
��1��B�Ļ�ѧʽ
(2��������ͼ��ʾ��ʵ��װ�ý���C��ˮ�ķ�Ӧ���ش������й�ͬ�⣺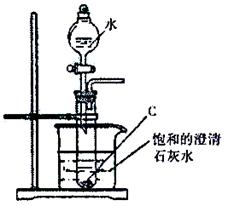
�� ����C��ˮ��Ӧ����D�Ļ�ѧ����ʽΪ�� ��
�� ���鵼�ܳ�������ķ���Ϊ�� ��
���ձ��е�����Ϊ�� ��
��3)�ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X��Ϊ����X�����ʣ��������ͼ��ʾʵ��װ�ã�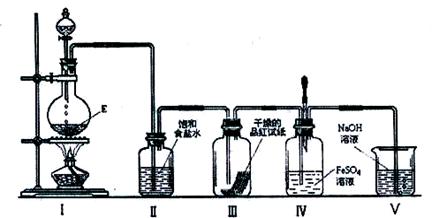
��ʵ������У��۲쵽װ�â��е�Ʒ����ֽ�ĺ�ɫ��ȥ����δ�۲쵽�������Ա仯����һԤ������Ϊ�˴ﵽ��һԤ����������ΪӦ����θĽ���
��
��ʵ�������װ�â��пɹ۲쵽�������������������������������������μ����ν�ͷ�ι��е��Լ����۲쵽��Һ��Ѫ��ɫ���йص����ӷ���ʽΪ��
, ��������������������������������������
��X��һ����Ҫ�Ļ���ԭ�ϣ��û�ѧ����ʽ��ʾX�ڻ��������ϵ�һ����;��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��ˮ�и���ģ�⣨5�£����Ի�ѧ�Ծ��������棩 ���ͣ��ƶ���
��ÿ��2�֣���18�֣�����A��B��C��D��E�������ʣ�����������ת����ϵ������EΪ��ɫ��ĩ������ͼת���о�������
��1��B�Ļ�ѧʽ
(2��������ͼ��ʾ��ʵ��װ�ý���C��ˮ�ķ�Ӧ���ش������й�ͬ�⣺
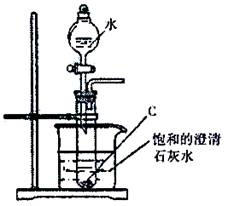
�� ����C��ˮ��Ӧ����D�Ļ�ѧ����ʽΪ�� ��
�� ���鵼�ܳ�������ķ���Ϊ�� ��
���ձ��е�����Ϊ�� ��
��3)�ڼ��������£�ij�����ᣨ����A�е�һ��Ԫ�أ���Ũ��Һ��E��Ӧ�����ɵ�������X��Ϊ����X�����ʣ��������ͼ��ʾʵ��װ�ã�
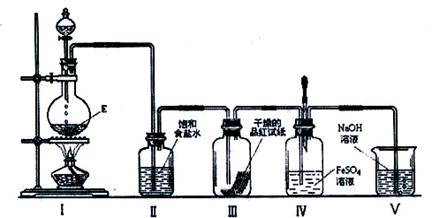
��ʵ������У��۲쵽װ�â��е�Ʒ����ֽ�ĺ�ɫ��ȥ����δ�۲쵽�������Ա仯����һԤ������Ϊ�˴ﵽ��һԤ����������ΪӦ����θĽ���
��
��ʵ�������װ�â��пɹ۲쵽�������������������������������������μ����ν�ͷ�ι��е��Լ����۲쵽��Һ��Ѫ��ɫ���йص����ӷ���ʽΪ��
, ��������������������������������������
��X��һ����Ҫ�Ļ���ԭ�ϣ��û�ѧ����ʽ��ʾX�ڻ��������ϵ�һ����;��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com