
| NaOHŅŅ“¼ | ”÷ |

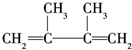
 £¬F1ŗĶF2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬¼Ó³ÉHBrŹ±F1ÓŠĮ½ÖּӳɲśĪļ£¬F2Ö»ÓŠŅ»ÖּӳɲśĪļ£¬G1ŗĶG2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壮ĒėĢīæÕ£ŗ
£¬F1ŗĶF2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壬¼Ó³ÉHBrŹ±F1ÓŠĮ½ÖּӳɲśĪļ£¬F2Ö»ÓŠŅ»ÖּӳɲśĪļ£¬G1ŗĶG2»„ĪŖĶ¬·ÖŅģ¹¹Ģ壮ĒėĢīæÕ£ŗ

 £¬EÓėŹŹĮæBr2·“Ó¦Ź±ÓŠĮ½Öּӳɷ½Ź½£¬¼“1£¬2¼Ó³ÉÉś³É
£¬EÓėŹŹĮæBr2·“Ó¦Ź±ÓŠĮ½Öּӳɷ½Ź½£¬¼“1£¬2¼Ó³ÉÉś³É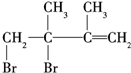 ŗĶ1£¬4¼Ó³ÉÉś³É
ŗĶ1£¬4¼Ó³ÉÉś³É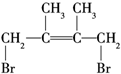 £¬ŌŁ¼Ó³ÉHBrŹ±F1ÓŠĮ½ÖּӳɲśĪļ£¬F2Ö»ÓŠŅ»ÖּӳɲśĪļ£¬ŌņF2ĪŖ
£¬ŌŁ¼Ó³ÉHBrŹ±F1ÓŠĮ½ÖּӳɲśĪļ£¬F2Ö»ÓŠŅ»ÖּӳɲśĪļ£¬ŌņF2ĪŖ £¬G1ĪŖ
£¬G1ĪŖ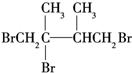 £¬½įŗĻÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ½ā“šøĆĢā£®
£¬½įŗĻÓŠ»śĪļµÄ½į¹¹ŗĶŠŌÖŹ½ā“šøĆĢā£® £¬EÓėŹŹĮæBr2·“Ó¦Ź±ÓŠĮ½Öּӳɷ½Ź½£¬¼“1£¬2¼Ó³ÉÉś³É
£¬EÓėŹŹĮæBr2·“Ó¦Ź±ÓŠĮ½Öּӳɷ½Ź½£¬¼“1£¬2¼Ó³ÉÉś³É ŗĶ1£¬4¼Ó³ÉÉś³É
ŗĶ1£¬4¼Ó³ÉÉś³É £¬ŌŁ¼Ó³ÉHBrŹ±F1ÓŠĮ½ÖּӳɲśĪļ£¬F2Ö»ÓŠŅ»ÖּӳɲśĪļ£¬ŌņF2ĪŖ
£¬ŌŁ¼Ó³ÉHBrŹ±F1ÓŠĮ½ÖּӳɲśĪļ£¬F2Ö»ÓŠŅ»ÖּӳɲśĪļ£¬ŌņF2ĪŖ £¬G1ĪŖ
£¬G1ĪŖ £¬
£¬ £¬¹Ź“š°øĪŖ£ŗ
£¬¹Ź“š°øĪŖ£ŗ £®
£®

 ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
ŠĀĖ¼Ī¬¼ŁĘŚ×÷Ņµŗ®¼Ł¼ŖĮÖ“óѧ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| Ļ©Ąą»ÆŗĻĪļ | Ļą¶ŌĖŁĀŹ |
| £ØCH3£©2C=CHCH3 | 10.4 |
| CH3CH=CH2 | 2.03 |
| CH2=CH2 | 1.00 |
| CH2=CHBr | 0.04 |




 £®ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉĪļµÄ½į¹¹¼ņŹ½
£®ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉĪļµÄ½į¹¹¼ņŹ½

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

 £¬F·Ö×ÓÖŠÖ»ÓŠĮ½øöäåŌ×Ó£¬²¢ĒŅ½ÓŌŚ²»ĻąĮŚµÄĢ¼Ō×ÓÉĻ£®
£¬F·Ö×ÓÖŠÖ»ÓŠĮ½øöäåŌ×Ó£¬²¢ĒŅ½ÓŌŚ²»ĻąĮŚµÄĢ¼Ō×ÓÉĻ£®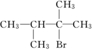



 +2NaOH
+2NaOH| ŅŅ“¼ |
| ”÷ |
 +2NaOH
+2NaOH| ŅŅ“¼ |
| ”÷ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğÕć½Ź”Äž²ØŹŠ°ĖŠ£øßŅ»ĻĀѧʌʌĩĮŖæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©£Ø1£©ĻĀ±ķĪŖĻ©Ąą»ÆŗĻĪļÓėäå·¢Éś¼Ó³É·“Ó¦µÄĻą¶ŌĖŁĀŹ£ØŅŌŅŅĻ©ĪŖ±ź×¼£©”£
| Ļ©Ąą»ÆŗĻĪļ | Ļą¶ŌĖŁĀŹ |
| (CH3)2C=CHCH3 | 10.4 |
| CH3CH=CH2 | 2.03 |
| CH2=CH2 | 1.00 |
| CH2=CHBr | 0.04 |
 ”£ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉĪļµÄ½į¹¹¼ņŹ½ ”£
”£ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉĪļµÄ½į¹¹¼ņŹ½ ”£²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2014½ģÕć½Ź”Äž²ØŹŠ°ĖŠ£øßŅ»ĻĀѧʌʌĩĮŖæ¼»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø9·Ö£©£Ø1£©ĻĀ±ķĪŖĻ©Ąą»ÆŗĻĪļÓėäå·¢Éś¼Ó³É·“Ó¦µÄĻą¶ŌĖŁĀŹ£ØŅŌŅŅĻ©ĪŖ±ź×¼£©”£
|
Ļ©Ąą»ÆŗĻĪļ |
Ļą¶ŌĖŁĀŹ |
|
(CH3)2C=CHCH3 |
10.4 |
|
CH3CH=CH2 |
2.03 |
|
CH2=CH2 |
1.00 |
|
CH2=CHBr |
0.04 |
ĻĀĮŠ»ÆŗĻĪļÓėäå¼Ó³ÉŹ±£¬Č”“ś»ł¶ŌĖŁĀŹµÄÓ°ĻģÓė±ķÖŠ¹ęĀÉĄąĖĘ£¬ĘäÖŠ·“Ó¦ĖŁĀŹ×īæģµÄŹĒ_______________£ØĢīŠņŗÅ£©£»
A£®(CH3)2C=C(CH3)2 B£®CH3CH=CHCH2CH3 C£®CH2=CH CH3 D£®CH2=CHBr
£Ø2£©0.5molÄ³Č²Ģž×ī¶ąÄÜÓė1molHCl·¢Éś¼Ó³É·“Ó¦µĆµ½ĀČ“śĢž£¬Éś³ÉµÄĀČ“śĢž×ī¶ąÄÜÓė3mol Cl2·¢ÉśČ”“ś·“Ó¦£¬Éś³ÉÖ»ŗ¬C”¢ClĮ½ÖÖŌŖĖŲµÄ»ÆŗĻĪļ”£ŌņøĆĢžµÄ½į¹¹¼ņŹ½ŹĒ £»
£Ø3£©Ä³·¼ĻćĢžA£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ104£¬Ģ¼µÄÖŹĮæ·ÖŹżĪŖ92.3£„”£
¢ŁA·Ö×ÓÖŠæÉÄܹ²Ę½ĆęµÄĢ¼Ō×Ó×ī¶ąÓŠ øö£»
¢Ś·¼ĻćĢžAŌŚŅ»¶ØĢõ¼žĻĀæÉÉś³É¼Ó¾Ūøß·Ö×Ó£¬øĆøß·Ö×Ó½į¹¹ÖŠµÄĮ“½ŚĪŖ £»
¢ŪŅ»¶ØĢõ¼žĻĀ£¬AÓėĒāĘų·“Ó¦£¬µĆµ½µÄ»ÆŗĻĪļÖŠĢ¼µÄÖŹĮæ·ÖŹżĪŖ85.7£„£¬Š“³öŠĪ³ÉøĆ»ÆŗĻĪļµÄÓŠ»ś·“Ó¦·½³ĢŹ½ £»
¢ÜŅŃÖŖ ”£ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉĪļµÄ½į¹¹¼ņŹ½ ”£
”£ĒėŠ“³öAÓėĻ””¢ĄäµÄKMnO4ČÜŅŗŌŚ¼īŠŌĢõ¼žĻĀ·“Ӧɜ³ÉĪļµÄ½į¹¹¼ņŹ½ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2013½ģɽ¶«¹ŚĻŲĪäѵøßÖŠø߶žĻĀѧʌµŚ¶ž“Īæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£© Ļ©ĢžAŌŚŅ»¶ØĢõ¼žĻĀæÉŅŌ°“ĻĀĆęµÄæņĶ¼½ųŠŠ·“Ó¦£®

ŅŃÖŖ£ŗDŹĒ £¬F·Ö×ÓÖŠÖ»ÓŠĮ½øöäåŌ×Ó£¬²¢ĒŅ½ÓŌŚ²»ĻąĮŚµÄĢ¼Ō×ÓÉĻ”£
£¬F·Ö×ÓÖŠÖ»ÓŠĮ½øöäåŌ×Ó£¬²¢ĒŅ½ÓŌŚ²»ĻąĮŚµÄĢ¼Ō×ÓÉĻ”£
ĒėĢīæÕ£ŗ
(1)CµÄ½į¹¹¼ņŹ½ŹĒ£ŗ___________£¬AŌŚŅ»¶ØĢõ¼žĻĀæÉÉś³Éøß·Ö×ÓG£¬GµÄ½į¹¹¼ņŹ½ŹĒ_________________________________ ”£
(2)æņĶ¼ÖŠŹōÓŚČ”“ś·“Ó¦µÄŹĒ£ŗ (ĢīŹż×Ö“śŗÅ)£¬ŹōÓŚ¼Ó³É·“Ó¦µÄŹĒ (ĢīŹż×Ö“śŗÅ).
(3)Š“³öÓÉD”śEµÄ»Æѧ·½³ĢŹ½______________________________________£»
Š“³öÓÉE”śFµÄ»Æѧ·½³ĢŹ½______________________________________
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com