

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

����գ�
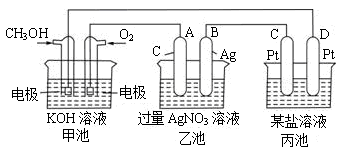
(1)���ʱ����ԭ��صĸ������Դ________���������������ĵ缫��ӦΪ_____________��
(2)�ŵ�ʱ�������ĵ缫��ӦʽΪ__________________________________________��
(3)�ڴ˹���������ȫ��Ӧ���ҳ���A������������648 g����׳�������������O2_______L��(��״����)
(4)���ڳ��³�ѹ�£�1 g CH3OHȼ������CO2��Һ̬H2Oʱ����22.68 kJ,��ʾ�÷�Ӧ���Ȼ�ѧ����ʽΪ ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���Դ��ȱ������������ٵ��ش����⡣�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������ע����ԭ�Ӿ����ԡ���ָ���ڻ�ѧƷ�ϳɹ����У��ϳɷ�������Ӧ����Ƴ��ܰѷ�Ӧ���������õ�����ԭ���Ͼ����ܶ��ת�������ղ����У�
��1����ҵ��һ������������ַ�Ӧ�ϳɼ״�
��ӦI�� CO(g) �� 2H2(g) CH3OH(g) ��H1
��ӦII�� CO2(g) �� 3H2(g) CH3OH(g) + H2O(g) ��H2
������Ӧ���ϡ�ԭ�Ӿ��á�ԭ����ǣ� ���I������
��2����֪�ڳ��³�ѹ�£�
�� 2CH3OH(l) �� 3O2(g) �� 2CO2(g)�� 4H2O(g) ��H ����1275.6 kJ��mol-1
�� 2CO (g)+ O2(g)�� 2CO2(g) ��H ����566.0 kJ��mol-1
�� H2O(g) �� H2O(l) ��H �� ��44.0kJ��mol-1
��д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ
__________________________________________________
��3���״�������ȼ�ϵ�أ��������Һ�ǣ�20%��30%��KOH��Һ����д���״�������ȼ�ϵ�طŵ�ʱ�����ĵ缫��Ӧʽ��
������
��4����ͼ��һ���绯ѧ����ʾ��ͼ��
�� пƬ�Ϸ����ĵ缫��ӦʽΪ ��
�� ����ʹ�ü״�������ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯96g����ȼ�ϵ����������Ҫ mol�״���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(7��)�ݱ�����Ħ��������˾������һ���Լ״�Ϊԭ�ϣ���![]() Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH2K2CO3+6H2O
����գ�
��1���ŵ�ʱ�������ĵ缫��ӦʽΪ____ _____��
��2�����ʱ����ԭ��صĸ������Դ_________��������
�������ĵ缫��ӦΪ__________________��
��3���ڴ˹���������ȫ��Ӧ���ҳ���B������������648g����׳�������������![]() _________L����״���£���
_________L����״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣��£�N2H4���ֳ���������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϡ���֪��25�棬101kPaʱ��32.0gN2H4����������ȫȼ�����ɵ������ų�����624kJ
��1��д����״̬��N2H4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ
������������ ��
��2���¡�����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH��Һ���¡�����ȼ�ϵ�طŵ�ʱ�������ĵ缫��Ӧʽ��
������
�� 3����ͼ��һ���绯ѧ����ʾ��ͼ��
�� пƬ�Ϸ����ĵ缫��Ӧʽ�� ��
�� ����ʹ���¡�����ȼ�ϵ����Ϊ�������еĵ�Դ��ͭƬ�������仯128g������һ����ȼ�ϵ����������Ҫ��״���µĿ���
L����������������������Ϊ20%��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�걱�����и߶���ѧ����ĩ���Ի�ѧ���� ���ͣ������
(7��)�ݱ�����Ħ��������˾������һ���Լ״�Ϊԭ�ϣ��� Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH
Ϊ����ʵ������ֻ��Ŀɳ��ĸ�Чȼ�ϵ�أ���һ�ε������ʹ��һ���¡���ͼ��һ���绯ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��2CH3OH+3O2+4KOH 2K2CO3+6H2O
2K2CO3+6H2O

����գ�
��1���ŵ�ʱ�������ĵ缫��ӦʽΪ____ _____��
��2�����ʱ����ԭ��صĸ������Դ_________��������
�������ĵ缫��ӦΪ__________________��
��3���ڴ˹���������ȫ��Ӧ���ҳ���B������������648g����׳������������� _________L����״���£���
_________L����״���£���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com