
ij����С��������CuO��NH3��Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ����������ʵ��װ�ã��г�װ��δ����������ʵ�顣��ش��������⣺
��1������a������Ϊ________������b�п�ѡ����Լ�Ϊ________��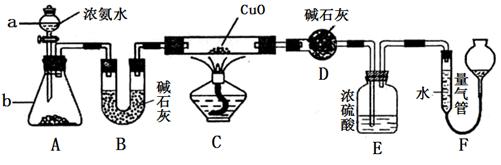
��2��ʵ�������������и������ʣ�������Ȫʵ�飬���ܳɹ����ǣ� ��
| A��Cl2�뱥��ʳ��ˮ | B��CO2 ��40%��NaOH��Һ |
| C��NH3�뱥��ʳ��ˮ | D��HCl��ˮ |
��1����Һ©�� �����ƻ��������ƻ��ʯ��
��2��A
��3��3CuO + 2NH3 3Cu + N2�� + 3H2O
3Cu + N2�� + 3H2O
��4������δ��Ӧ�İ�������ֹF��ˮ��������D
��5��9n/11.2m
���������������1��ʵ�����г���Ũ��ˮ���Ȼ�Ũ��ˮ�μӵ����������ƻ����������ϣ����������ƻ��������Ƶ���ˮ�Լ��ܽ��������ʹ�����������2��������Ȫʵ��ɹ��Ĺؼ���������Һ�����ܽ�ȴ���������㹻���ѹǿ�Cl2�ڱ���ʳ��ˮ�ܽ���٣������γ�ѹǿ�������Ȫʵ�飬���ܳɹ�������ѡ����ԡ���3��NH3��CuO����ʱ����������ԭ��Ӧ�ķ���ʽ�ǣ�3CuO + 2NH3 3Cu + N2�� + 3H2O��4��Eװ����Ũ���������������δ��Ӧ�İ�������ֹF��ˮ��������D . ��5�������D����mg��������ˮ������������mg��װ��F�����������ΪnL������N2�ڱ�״�������nL��n(H2O):n(N2)=(mg��18g/mol):(nL��22.4L/mol)="11.2m:9n." �����е������ԭ�Ӹ�����Ϊ(9n��2): (11.2m��2)= 9n/11.2m
3Cu + N2�� + 3H2O��4��Eװ����Ũ���������������δ��Ӧ�İ�������ֹF��ˮ��������D . ��5�������D����mg��������ˮ������������mg��װ��F�����������ΪnL������N2�ڱ�״�������nL��n(H2O):n(N2)=(mg��18g/mol):(nL��22.4L/mol)="11.2m:9n." �����е������ԭ�Ӹ�����Ϊ(9n��2): (11.2m��2)= 9n/11.2m
���㣺����������ʶ��Ӧ�úͰ�����ʵ�����Ʒ������ʵ�֪ʶ��


 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д� ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������������������Ź㷺��Ӧ�á�ij��ѧ��ȤС������ͼһװ��̽����������
�����ʡ�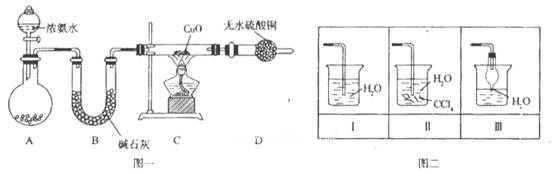
��1��װ��A����ƿ���Լ���ѡ��__________������ţ���B��������_______��
a����ʯ�� b��Ũ���� C����ʯ�� d���ռ���Һ
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ��Ȼ��Ӧ��_______����I���
I�������������Բ����ƿ�м��백ˮ
����װ��C
��3��ʵ���й۲쵽C��CuO��ĩ��죬D����ˮ����ͭ���������ռ���һ�ֵ������壬��÷�Ӧ��ػ�ѧ����ʽΪ__________________________________________________�� �÷�Ӧ֤����������_________ �ԡ�
��4����ʵ��ȱ��β������װ�ã�ͼ��������������β����װ����_________����װ����ţ���
��5��������������ˮ������״���£���2.24 L�İ�������ˮ���0.5 L��Һ��������Һ�����ʵ���Ũ��Ϊ________________mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ��ʵ������ȡ���ռ�Cl2��װ�á�A��Cl2����װ�ã���E��Ӳ�ʲ�������װ��ͭ˿����FΪ����Ĺ��ƿ���ձ�GΪβ������װ�á�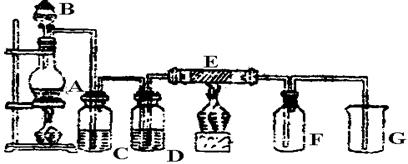
�Իش�
��1��A�з����Ļ�ѧ��Ӧ�����ӷ���ʽΪ ��
��2��C��G�и�װ��ҩƷ��C__________��G__________��
��3��E��Ӧ����ʽΪ ��
��4��д��G�з�Ӧ�����ӷ���ʽ ��
��5������Cl2��SO2�����ʵ������Ư�����ü������û�ѧ����ʽ����ԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС����ʵ������ȡƯ�ۣ���̽��������ʯ���鷴Ӧ�������Ͳ��
��֪���ٶ���������Ũ���ᷴӦ���Ʊ�������ͬʱ����MnCl2��
�������ͼ�ķ�Ӧ�Ĺ����зų��������¶Ƚϸ�ʱ�������ͼ�ܷ������·�Ӧ��6Cl2 + 6Ca(OH)2 5CaCl2 + Ca(ClO3)2 + 6H2O
5CaCl2 + Ca(ClO3)2 + 6H2O
����ȤС�����������ʵ��װ�ã�����ʵ�顣
�� �� �� ��
��ش��������⣺
��1���ټ�װ���з�����Ӧ�Ļ�ѧ����ʽ�� ��
����װ���е��Լ��� �������� ��
�۸���ȤС����300mL 12mol/L������17.4g MnO2�Ʊ��������������������������ʯ���鷴Ӧ���������������Ƶñ�������� L��Ca(ClO)2 g��
��2��С���Ա���֣�������Ca(ClO)2����������С������ֵ���������ۺ���Ϊ����������δ��ʯ���鷴Ӧ���ݳ����Լ��¶������ǿ���ԭ��Ϊ��̽����Ӧ�����Բ����Ӱ�죬������ȡһ������ʯ���飬���������ٵ�ͨ�������������ó���ClO����ClO3�� �������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���Ĺ�ϵ���ߣ����Ա�ʾΪ��ͼ��������������ˮ�ķ�Ӧ����
��ͼ�����ߢ��ʾ ���ӵ����ʵ����淴Ӧʱ��仯�Ĺ�ϵ��
����ȡʯ�����к���Ca(OH)2�����ʵ���Ϊ mol��
����ȡһ����ڵ����ʵ���Ca(OH)2��ʯ���飬�Խϴ������ͨ��������������Ӧ���ò�����Cl�������ʵ���Ϊ0.35mol��������� = ��
= ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ��ѧʵ����ȤС��Ϊ��̽����ʵ�����Ʊ�Cl2�Ĺ����� ��ˮ������HCl�ӷ�������ͬʱ֤��������ijЩ���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ��(֧���õ�����̨ʡ��)������������⡣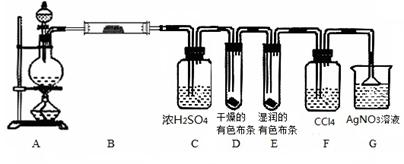
��1���� MnO2��Ũ�����Ϲ����Ƶ������Ļ�ѧ����ʽ��____ ____��
��2�����ú���0��2 mol HCl��Ũ������������MnO2��Ӧ��Cl2���Ƶõ�Cl2����ڱ�״��������С��1��12 L��ԭ����_____________________________________________��
��3����װ��B��������____________________________________________________��
װ��B�е�������____________________________��
��װ��D��E���ֵIJ�ͬ����˵����������_____________________________��
��װ��F��������________________________��
��װ��G�з�����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ����ȡ��ˮ�Ȼ�ͭ��ʵ��װ��ͼ����Ũ����μӵ�ʢ�ж������̷�ĩ��Բ����ƿ�С���ش��������⣺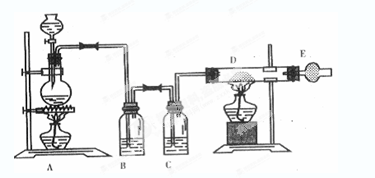
(1)ʢ��Ũ�������������Ϊ�ߣߣߣ���
(2)��ƿ�з�����Ӧ�Ļ�ѧ����ʽ ��
(3)Cƿ�е��Լ����ߣߣߣ������������ߣߣߣ���
(4)������D�з�����Ӧ�Ļ�ѧ����ʽ ����Ӧ�������ߣߣ���
(5)�����E��ʢ�м�ʯ��(CaO+NaOH)�����������ߣߣߣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ͼ��ʾװ�ÿ������һϵ��ʵ�飨ͼ�мг�װ������ȥ��
��ش��������⣺
I����1������p��������_________________������װ��A��Ũ������������ƹ�����ȡSO2���壬��ͨ��װ��B��ɱ������ʵ�飬����д���пհף�
| B������� | �� | �� | �� |
| ��պ�Լ� | ʯ����Һ | Ʒ����Һ | ��ˮ����ɫ�� |
| ���� | | ��ɫ | |
| ����SO2������ | ˮ��Һ������ | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУ����С��ͬѧ���ʵ�飬̽��ľ̿��Ũ�����ڼ��������·�Ӧ��������ijɷ֡�
��ʵ��̽����
��1��ľ̿��Ũ���ᷴӦ�Ļ�ѧ����ʽ��C+2H2SO4��Ũ�� CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
��2��Aװ����Ʒ����Һ��ɫ �����ɫ������ɫ������֤������ ���塣
��3��ʵ������У�װ��C���۲쵽��������_______________________________��
��ʵ�����ۡ�
��4����ͬѧ��Bװ���ܷ����SO2���������塣����ΪӦ����B��Cװ��֮��������ͼ�� װ�ã���ȷ��SO2�Ƿ������
����ϵʵ�ʡ�
��5��ú��ʯ�͵�ȼ�չ����ж��ж�������Ͷ�����̼�ŷţ����ж���������ɵĻ���Ӱ����Ҫ��_________��������̼��ɵĻ���Ӱ����Ҫ��_______����ÿ�ո�ֻ��һ��ѡ�
A������ B���ƻ������� C������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
ij�����ĩ���п��ܺ���K2CO3��KNO3��NaNO2��K2SO3��Na2SO4��FeO��Fe2O3�е������֣�ijͬѧΪȷ���ù����ĩ�ijɷ֣�ȡ��������ʵ�飬ʵ����̼��������£� 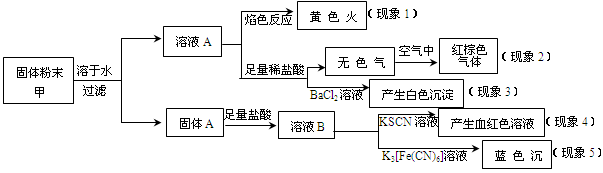
��ͬѧ�ó��Ľ�����ȷ����
| A����������1���Ƴ��ù����ĩ�к�����Ԫ�أ���������Ԫ�� |
| B����������2���Ƴ��ù����ĩ��һ������NaNO2 |
| C����������3���Ƴ��ù����ĩ��һ������Na2SO4 |
| D����������4������5���Ƴ��ù����ĩ��һ������FeO��Fe2O3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com