
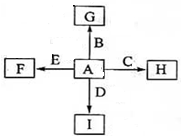
 ��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G
��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G
| ||
| ||
| ||
| ||
| ||
| ||
| ||
| ||


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪���ٷ�ӦC+G
��ͼ�У�A��B��C��D��E�ǵ��ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪���ٷ�ӦC+G| ���� |
| ||
. |
| ||
| ||
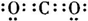
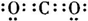
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�긣��ʡ������һ�и������Ĵ��������ۻ�ѧ�Ծ��������棩 ���ͣ������
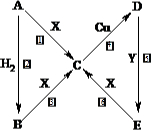
��ͼ�У�A��B��C��D��E�ǵ���G��H��I��F��B��C��D��E�ֱ��A�γɵĶ�Ԫ�������֪��
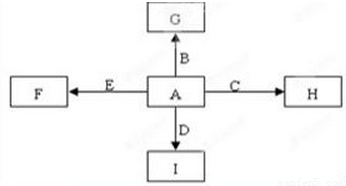
����GΪ����ɫ���壬��ӦC��G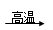 B��H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B��H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
��I��һ�ֳ������������壬����E���Է�����Ӧ��2E��I 2F��D��F��EԪ�ص���������Ϊ60%���ش����⣺
2F��D��F��EԪ�ص���������Ϊ60%���ش����⣺
��1�����з�Ӧ�Ļ�ѧ����ʽΪ______________________________��
��2��3.2 g G�������ᣬ�õ�����Һ��ͭ����ȫ��Ӧ��������������ͭ�۵�����Ϊ
��3��C�����NaOH��Һ��Ӧ�����ӷ���ʽΪ______________________��
��4��E��I��ȼ�չ۲쵽��������___________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G
��ͼ�У�A��B��C��D��E�dz������ʣ�G��H��I��F��B��C��D��E�ֱ��A�γɵĻ������֪���ٷ�Ӧ2C+G 2B+H�����ų��������ȣ��÷�Ӧ����������ĺ��ӣ���I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I
2B+H�����ų��������ȣ��÷�Ӧ����������ĺ��ӣ���I��һ�ֳ������������壬����E���Է�����Ӧ��2E+I 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com