
ijͬѧ������һƿ��������84����Һ��������������Ϻ�����Һ��װ˵���õ�������Ϣ��
��84����Һ������25%NaClO 1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á�
�����������Ϣ�����֪ʶ�ش��������⣺
(1)����84����Һ�������ʵ���Ũ��Ϊ________ mol��L��1��
(2)��ͬѧȡ100 mL����84����Һ��ϡ�ͺ�����������ϡ�ͺ����Һ��c(Na��)��________ mol��L��1(����ϡ�ͺ���Һ�ܶ�Ϊ1.0 g��cm��3)��
(3)ijʵ������480 mL��25%NaClO������Һ����ͬѧ���ĸ���84����Һ�����䷽������NaClO�������Ƹ�����Һ��
������˵����ȷ����________��

A������ͼ��ʾ�������У��������Dz���Ҫ�ģ�����һ�ֲ�������
B������ƿ������ˮϴ����Ӧ��ɲ���������Һ����
C�����ù������ƷNaClO�����ƿ��ܵ��½��ƫ��
D����Ҫ������NaClO��������Ϊ143 g
�������ƹ����У����в�������ʹ���Ƶ���Һ��Ũ��ƫ�����________��
A���ձ�����Һת�Ƶ�����ƿ��ʱ��δϴ���ձ�
B������ʱ�����ӿ̶���
C������ʱ�����ӿ̶���
D����Һʱ��������Һ�彦��
��(1)4.0��(2)0.04��(3)��C����B
����������(1)����c��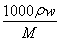 ��c(NaClO)��4��0 mol��L��1��
��c(NaClO)��4��0 mol��L��1��
(2)ϡ��100����Ũ��Ϊԭ����1%��
(3)��ѡ��A������������ƽ����NaClO���壬�����ձ����ܽ�NaClO�����ò��������н������������������ƿ�ͽ�ͷ�ι������ݣ�ͼʾ�е�����������������Ҫ�������貣�����ͽ�ͷ�ιܡ�ѡ��B�����ƹ�������Ҫ����ˮ�����Ծ�ϴ�Ӹɾ�������ƿ���غ�ɺ���ʹ�á�ѡ��C������NaClO�����տ����е�H2O��CO2�����ʣ�������ƷNaClO���ܲ��ֱ��ʵ���NaClO���٣����Ƶ���Һ�����ʵ����ʵ�����С�����ƫ�͡�ѡ��D��Ӧѡȡ500 mL������ƿ�������ƣ�Ȼ��ȡ��480 mL���ɣ�������ҪNaClO��������0.5 L��4.0 mol��L��1��74.5 g��mol��1��149 g��
����c�� �жϣ�A��Dѡ����ʹnƫС��Ũ��ƫС��Bѡ���и��ӿ̶��ߣ�ʹVƫС��Ũ��ƫ��Cѡ�������ӿ̶��ߣ�ʹVƫ��Ũ��ƫС��
�жϣ�A��Dѡ����ʹnƫС��Ũ��ƫС��Bѡ���и��ӿ̶��ߣ�ʹVƫС��Ũ��ƫ��Cѡ�������ӿ̶��ߣ�ʹVƫ��Ũ��ƫС��


 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��8���绯ѧ��ϰ���������棩 ���ͣ������
�ס��ҵ�ʵ��װ����ͼ��ʾ���������ֱ����ȼҵ����ʾ��ͼ���Ʊ������ѵ�ʾ��ͼ��

��ش��������⣺
(1)д����װ����̼������ĵ缫��Ӧʽ��_______________________________��
(2)��֪��5Cl2��I2��6H2O=10HCl��2HIO3������ʪ��ĵ���?KI��ֽ������װ���е�̼������������Ϊ________________________________������װ����ת��0.02 mol���Ӻ�ֹͣʵ�飬�ձ�����Һ�����Ϊ200 mL�����ʱ��Һ��pH��________��(���������£��Ҳ����ǵ���������Ӧ)
(3)��ҵ�Ͼ����õ����ӽ���Ĥ�����ӽ���Ĥ�������ӽ���Ĥ�������ӽ���Ĥ���֣������ӽ���Ĥֻ����������ͨ���������ӽ���Ĥֻ����������ͨ��������װ���еķ�Ӧ���ڹ�ҵ����ʱ��Ϊ����ֹ��������֮��ķ�Ӧ��ͨ�������ͼ��ʾ��װ�ã�Na�����ƶ�������ͼ�б�ע����H2�ij�����________(����C������D������E������F��)��________(������������������)�������ӽ���Ĥ���������ӽ���Ĥ��
(4)�о����֣�������ʯī������������������������CaO������ʣ����ö�װ�û�ý����ƣ����Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
��д�������ĵ缫��Ӧʽ��___________________________________________��
�����Ʊ�������ǰ��CaO���������䣬��ԭ����(���ϻ�ѧ�������)__________________________________________________________��
�����������趨�ڸ����������ϵ�ԭ����____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��5����ѧ��Ӧ��������ϰ���������棩 ���ͣ�ѡ����
100 g̿��ȼ�����������У�COռ ��CO2ռ
��CO2ռ ����C(s)��
����C(s)�� O2(g)=CO(g)����H����110.35 kJ��mol��1��CO(g)��
O2(g)=CO(g)����H����110.35 kJ��mol��1��CO(g)�� O2(g)=CO2(g)����H����282.57 kJ��mol��1������Щ̿����ȫȼ�������ʧ�������� (����)��
O2(g)=CO2(g)����H����282.57 kJ��mol��1������Щ̿����ȫȼ�������ʧ�������� (����)��
A��784.92 kJ B��2 489.44 kJ C��1 569.83 kJ D��3 274.3 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��3��������Ҫ�Ļ�ѧ��Ӧ��ϰ���������棩 ���ͣ������
(1)���ڵ��۵⻯����Һ�У��μ������������Ƽ�����Һ�������ῴ����Һ����ɫ��������Ϊ________�����ӷ���ʽ__________________________��
���ڵ�͵����γɵ���ɫ��Һ�У��μ��������Ƽ�����Һ��������ɫ����ʧ��������Ϊ________________________�����ӷ���ʽ��_______________________��
���Ա�������ʵ�����õĽ������I2��ClO����SO42������������ǿ������˳������Ϊ________________________________________��
(2)������Ƭ��ͭƬ�����ʵ��֤��������ʵ����д����Ӧ�Ļ�ѧ����ʽ��
��Ũ����������Ա�ϡ����ǿ��___________________________��
���Ȼ�����Һ��Fe3���������Ա�����ͭ��Һ�е�Cu2��ǿ��__________________________________________��
�����Ļ�ԭ�Ա�ͭǿ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��3��������Ҫ�Ļ�ѧ��Ӧ��ϰ���������棩 ���ͣ�ѡ����
LiAlH4�ǽ���������������л��ϳ��еij����Լ�����ˮ�ܾ��ҷ�Ӧ�ͷų�������LiAlH4��125 ���ֽ�ΪLiH��H2��Al�����������������(����)��
A��LiAlH4����ȩ���������Ҵ���LiAlH4����ԭ��
B��LiAlH4��D2O��Ӧ������������Ħ������Ϊ4 g��mol��1
C��1 mol LiAlH4��125 ����ȫ�ֽ⣬ת��3 mol����
D��LiAlH4��ˮ��Ӧ������������ʱ����ѧ����ʽ�ɱ�ʾΪ��LiAlH4��4H2O=Al(OH)3��LiOH��4H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��2����ѧ���ü�����ϰ���������棩 ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ������������ȷ����(����)��

A��3 mol NF3��ˮ��ȫ��Ӧ����HNO3��NO��ת�Ƶ�����2NA
B����״���£�32 g��(�ṹ��ͼ)��SS����ĿΪNA
C����״����11.2 L�������������õ��Ӷ���Ϊ8NA
D����a mol��L��1 H���Ĵ�����Һϡ��10����c(H��)Ϊ0.1a mol��L��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��1�����ʵ�������ʺͷ�����ϰ���������棩 ���ͣ������
�������й����ʵ�ת����ϵͼ(���ֲ�����ʡ��)������AΪ���ʣ�E�ڳ�����ΪҺ�壬D��һ���������壬C����Է�������Ϊ78����ش�������⡣

(1)���ж�C�Ľṹ�������ƶ��в���ȷ����(����)��
A�������ڿ����л��ɰ�ɫ B������ǿ������
C�������д������Ӽ���Ǽ��Լ� D����һ�ּ���������
(2)A��ԭ�ӽṹʾ��ͼΪ____________________��H�ĵ���ʽΪ____________________��E�ĽṹʽΪ____________________��
(3)C��E��Ӧ����H�����ӷ���ʽΪ__________________����Ӧ���������뻹ԭ�������ʵ���֮��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��14�����ʽṹ��������ϰ���������棩 ���ͣ������
2012��12�£��ҹ��ɹ�ʵ�ּ���15�ɻ���������������ĸ�ϵ���ʵ�飬���캽ĸƽ̨�ͽ��طɻ��벻�����ѡ�����A�Ȳ��ϡ���ش��������⣺
(1)д���ѻ�̬ԭ�ӵĺ�������Ų�ʽ��___________________________��
(2)��֪Ԫ��A���ڵ�3���ڣ���ԭ�ӵĵ�һ�����ĵ��������������ʾ��
������(kJ/mol) | I1 | I2 | I3 | I4 |
A | 738 | 1 451 | 7 733 | 10 540 |
��A��Ԫ�ط���Ϊ________��
����Ԫ��Aͬ���ڵĵ縺������Ԫ��Ϊ________(��Ԫ�ط���)����Ԫ������ͬ���ڵ���һ�ַǽ���Ԫ���γ�5ԭ�ӷ��ӣ��÷��ӵ�����ԭ�Ӳ��õ��ӻ���ʽ��________���÷��ӵĿռ乹��Ϊ________��
(3)��þ�Ͻ�����������ܷɻ�����Ҫ���ϡ���֪�ѡ�þ�����������������ܶѻ�( )������˵����ȷ����________(�����)��
)������˵����ȷ����________(�����)��
A������þ�Ͻ�����������ܷɻ�����Ҫ������۸�������ķɻ��������ü�Ǯ
B���ڸ������и�����Ѳ�������κΰ�ȫ�¹�
C���ѡ�þ���������У�����λ����Ϊ12
D�������ѵ��۵�ܸ�(1 668 ��)�����������ϵ����
(4)��������������(��ͼ��ʾ)���������캽��ĸ���е��������������þ�����Aԭ��Ϊ�����Ķ��㣬A������Ca��Sr��Ba��Pb����B��V��Cr��Mn��Feʱ�����ֻ�������кܺõĵ绯ѧ���ܡ���A��B��O��ʾ�������������ᄃ��Ļ�ѧʽΪ________��
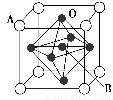
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��10������Ԫ�ؼ��仯������ϰ���������棩 ���ͣ�ѡ����
��ҵ����������(��Ҫ�ɷ�ΪAl2O3����Fe2O3����)Ϊԭ��ұ�����Ĺ����������£�

����������ȷ����(����)��
A���Լ�X����������������Һ��Ҳ����������
B����Ӧ�������˺����ó���Ϊ��������
C��ͼ����ʾת����Ӧ������������ԭ��Ӧ
D����Ӧ���Ļ�ѧ����ʽΪNaAlO2��CO2��2H2O=Al(OH)3����NaHCO3
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com