
| ||
| ||
| ||
| ||


 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
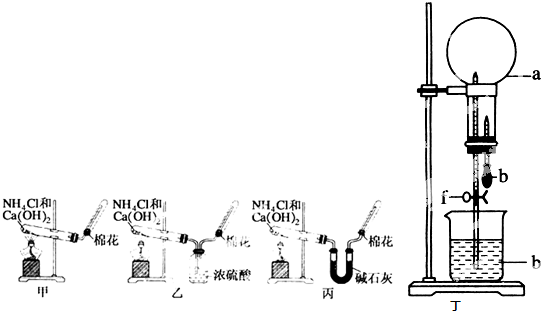
��Ŀ�����л�ѧ ��Դ��2011�꽭��ʡ������ѧ���촨��ѧ��һ1��������ѧ�Ծ� ���ͣ������
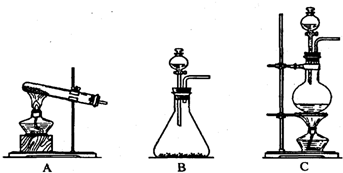
��10�֣��ס��ҡ�����λͬѧ�ֱ�����������ʵ��װ�ü���ѧҩƷ�����м�ʯ��Ϊ�����������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش��������⣺
��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��_______________________
��2����λͬѧ���������ſ������ռ���������������ˮ������ԭ����_________
A������������ˮ  B��������������ˮ
B��������������ˮ
C�������ܶȱȿ����� D�������ܶȱȿ���С
E�������ܶȱ�ˮ�� F�������ܶȱ�ˮС
��3����λͬѧ������װ����ȡ����ʱ,������ͬѧû���ռ����������ռ�������������Ҫԭ����____________________ (�û�ѧ����ʽ��ʾ)��
��4�����鰱���Ƿ��ռ����ķ�����_________
A���ŵ��а����ݳ�
B������������
C����ʪ��ĺ�ɫʯ����ֽ���Թܿڼ��飬������ֽ����
D����ʪ�����ɫʯ����ֽ���Թܿڼ��飬������ֽ���
��5����λͬѧ����Ϊ���ǵ�ʵ��װ��Ҳ�����ڼ���̼����粒�����ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���___________(��ס������ҡ�����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ�������и�һ��ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
�ס��ҡ�����λͬѧ�ֱ�����������ʵ��װ�ü���ѧҩƷ�����м�ʯ��Ϊ�����������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش��������⣺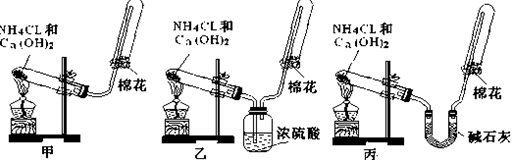
��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ�� ��
��2����λͬѧ���������ſ������ռ���������ԭ���� ��
��3����λͬѧ������װ����ȡ����ʱ,������һλͬѧû���ռ�������������ǵ�ʵ���������ȷ��������Ϊû���ռ���������ͬѧ�� ��(���ס������ҡ�����)���ռ�������������Ҫԭ���� (�û�ѧ����ʽ��ʾ)��
��4�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ� ��
��5����λͬѧ����Ϊ���ǵ�ʵ��װ��Ҳ�����ڼ���̼����粒�����ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���___________(��ס������ҡ�����)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�켪��ʡ���������ѧУ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
I�ס��ҡ�����λͬѧ�ֱ�����������ʵ��װ�ü���ѧҩƷ�����м�ʯ��Ϊ�����������ƺ���ʯ�ҵĻ�����ȡ�������������̽�������ش��������⣺
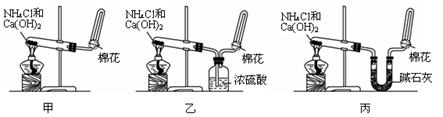
��1����λͬѧ��ȡ�����Ļ�ѧ����ʽΪ��_____________________________________________��
��2����λͬѧ���������ſ������ռ���������ԭ����__________________________________.
��3����λͬѧ������װ����ȡ����ʱ,������һλͬѧû���ռ�������������ǵ�ʵ���������ȷ��������Ϊû���ռ���������ͬѧ��___________��(���ס������ҡ�����)���ռ�������������Ҫԭ����_____________________________________(�û�ѧ����ʽ��ʾ)��
��4�����鰱���Ƿ��ռ����ķ����ǣ�������������������ͽ��ۣ�_______________________
__________________________________________________________________________________
_______________________________________________________________________________��
��5����λͬѧ����Ϊ���ǵ�ʵ��װ��Ҳ�����ڼ���̼����粒�����ȡ�����İ��������ж��ܹ��ﵽʵ��Ŀ�ĵ���___________(��ס������ҡ�����)����װ���е�NH4HCO3�����ܷ���NH4CL�������NH3��______________(��ܡ����ܡ�)��
II������ʾ�����������ڴ����а���ȼ�ա�������ijУ��ѧС��ѧ�������ͼװ��(ͼ�����еȼг�װ������ȥ)���а����������ڲ�ͬ�����·�Ӧ��ʵ�顣

��6����װ��A��ȡ����������İ��������Թ�����̼���Σ���ʯ�ҵ�������__________________________________��
��7���������İ��������������ͨ��װ��B(����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ����������Ļ�ѧ����ʽ��__________________���Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��_________________��
��8����������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У�����b���϶˵�ȼ������
��������ͨ����Ⱥ�˳����________����������_____________��
�ڰ���ȼ�յĻ�ѧ����ʽ��______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com