
| O | 2- 4 |
| n |
| V |
| n |
| V |
| 0.2mol |
| 0.4L |
| 0.3mol |
| 0.4L |
| 0.5mol/L��400mL |
| 0.2mol/L |
| 0.6mol |
| 1L |
| 0.5mol |
| 1L |


 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
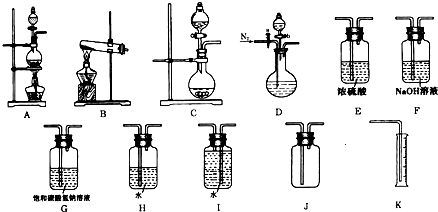
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣���һ����ͭ������Ũ�����ַ�Ӧ�����Եõ�6.72L NO2���壨��������ڱ�״���²ⶨ����
��1��д��ͭ��Ũ���ᷴӦ��ѧ����ʽ����������ת�Ƶķ������Ŀ
��2������Ӧ����Һ���Ϊ100mL����������Һ��Cu2+�����ʵ���Ũ���Ƕ��٣�
��3����������ˮ���ռ����壬����ռ�����������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�������ʡ��������У��һ��ѧ����ĩ������ѧ�Ծ� ���ͣ�������
��һ����ͭ������Ũ�����ַ�Ӧ�����Եõ�6.72L NO2���壨��������ڱ�״���²ⶨ����
��1��д��ͭ��Ũ���ᷴӦ��ѧ����ʽ����������ת�Ƶķ������Ŀ��
��2������Ӧ����Һ���Ϊ100mL����������Һ��Cu2+�����ʵ���Ũ���Ƕ��٣�
��3����������ˮ���ռ����壬����ռ�����������Ϊ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���������У��һ��ĩ������ѧ�Ծ� ���ͣ�������
��8�֣���һ����ͭ������Ũ�����ַ�Ӧ�����Եõ�6.72L NO2���壨��������ڱ�״���²ⶨ����
��1��д��ͭ��Ũ���ᷴӦ��ѧ����ʽ����������ת�Ƶķ������Ŀ
��2������Ӧ����Һ���Ϊ100mL����������Һ��Cu2+�����ʵ���Ũ���Ƕ��٣�
��3����������ˮ���ռ����壬����ռ�����������Ϊ���٣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com