
| A�� | ʵ����з�����Ӧ�����ӷ���ʽΪ��Fe2++Cl2�TFe3++2Cl-��Fe3++3SCN-�TFe��SCN��3 | |
| B�� | ͨ������ʵ���ȷ���û�����Ļ�ѧʽΪ����NH4��2Fe��SO4��2•6H2O | |
| C�� | ֻ��ʵ��٢ۢܣ�Ҳ�ܴﵽʵ��Ŀ�� | |
| D�� | Ϊ�˼���SO42-�����Խ����е��Լ���ΪHNO3�ữ��Ba��NO3��2 |
���� ��ȡ�����þ������Թ��У����ȣ������Թܿڴ���Һ�壬�Ҹ�Һ����ʹ��ˮ����ͭ������˵����ˮ���ɣ�
��ȡ�����þ������Һ���Թ��У�����KSCN��Һ��û�����Եı仯���μӼ�����ˮ����Һ�Ժ�ɫ��˵�������������ӣ�
��ȡ�����þ������Һ���Թ��У��ټ������ᣬû�����Եı仯���ټ���BaCl2��Һ���а�ɫ����������˵�������������
��ȡ�����þ������Һ���Թ��У�����ŨNaOH��Һ������ʹʪ��ĺ�ɫʯ����ֽ���������������ͬʱ���а�ɫ�����������ó����ܿ��ɻ���ɫ�������ձ�Ϊ���ɫ������˵������笠����������ӣ��Դ������
��� �⣺��ȡ�����þ������Թ��У����ȣ������Թܿڴ���Һ�壬�Ҹ�Һ����ʹ��ˮ����ͭ������˵����ˮ���ɣ�
��ȡ�����þ������Һ���Թ��У�����KSCN��Һ��û�����Եı仯���μӼ�����ˮ����Һ�Ժ�ɫ��˵�������������ӣ�
��ȡ�����þ������Һ���Թ��У��ټ������ᣬû�����Եı仯���ټ���BaCl2��Һ���а�ɫ����������˵�������������
��ȡ�����þ������Һ���Թ��У�����ŨNaOH��Һ������ʹʪ��ĺ�ɫʯ����ֽ���������������ͬʱ���а�ɫ�����������ó����ܿ��ɻ���ɫ�������ձ�Ϊ���ɫ������˵������笠����������ӣ�
A��Fe2++Cl2=Fe3++2Cl-�����ӷ�Ӧ����ʽδ��ƽ����A����
B������ʵ��õ���������ȷ�����е����ӣ���֤�����нᾧˮ����֤��������������ӣ���֤������笠����Ӻ��������ӣ����ݻ��ϼ۹�������Ϊ����NH4��2Fe��SO4��2•6H2O����B��ȷ��
C��ʵ�����֤���������ӵĴ��ڣ�����ʵ�����Ҳ֤�����������ӵĴ��ڣ��ʢڿ��Բ�������C��ȷ��
D������SO42-�������ų��������ӵ�Ӱ�죬�������е��Լ���ΪHNO3�ữ��Ba��NO3��2����ϡ��Һ�д������������Ҳ�ܳ��ִ�����D����
��ѡBC��
���� ���⿼��ѧ���������ӵļ��鷽����ע���������ӷ�Ӧ�����Ǽ������ӵĹؼ����ѶȲ���


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | C2H4 | B�� | CH3CH2OH | C�� | C6H6 | D�� | CH3COOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���������� | B�� | ��������� | C�� | ������Ӳ��� | D�� | ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | FeO��Fe2O3�Ļ���� | B�� | Fe2O3��Fe3O4�Ļ���� | ||
| C�� | CuO��Fe2O3�Ļ���� | D�� | CuO��Fe3O4�Ļ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
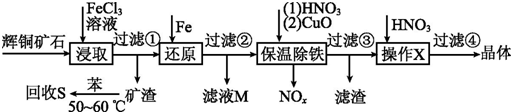
| ������ | Cu��OH��2 | Fe��OH��3 | Fe��OH��2 |
| ��ʼ����pH | 4.7 | 2.7 | 7.6 |
| ��ȫ����pH | 6.7 | 3.7 | 9.6 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com