
��13�֣�����ѧ����̼���ȡ�����ѧʽCOCl2������Ϣ�Զ���֮һ��
2-1 ������ѧ���ʻ��ã�������±�ĵ��ͷ�Ӧ������ˮ�⡢����ʹ��⡣
��1�������������Ҵ���Ӧ���ò���Ļ�ѧ������ ��
��2������������������Ӧ�Ļ�ѧ����ʽ ��
��3����������AlCl3�����������������Ӧ���ò���Ľṹ��ʽ ��
��4�������뱽����������Ӧ�ɵ������ᱽ�����м����Ľṹ��ʽ�� �������ᱽ���Ľṹ��ʽ�� ��
2-2 ʵ���ҿ������Ȼ�̼�ͷ������ᣨH2SO4?SO3����Ӧ�Ʊ�������д����ѧ����ʽ��
2-3 BTC��һ���ȶ��İ�ɫ�ᾧ�壬�۵�Ϊ78��82�棬�е�Ϊ203��206�档1mol BTC����һ�������·ֽ����3mol�����������ֱ���Ϊ��������������ҵ�Ͽ�������̼����������ȴ���Ӧ�Ʊ�BTC��BTC�ķ�Ӧ������������ƣ����Ժʹ���ȩ���������������ᡢ�ӡ��ǰ��ȶ��ֻ����ﷴӦ����˵Ͷ��Ե�BTC�ڻ�ѧ��Ӧ����ȫ������綾�������ã��Ĺ����ϳ���صĻ�����Ʒ��
��1��BTC������������ԭ�Ӷ��ȼۣ���д���ṹ��ʽ
��2���������⣬BTC�ȹ�������ʲô�ŵ㣿
2-4 2004��6��15�ո���ʡ���ʽṹ�о���������ﹹ����һ������ҵ����ʵ�������������ж�����й©����ɣ���������260������ҽԺ���Ρ��������Ȱ�����ʽ�ֽ⣺COCl2(g)��CO(g)��Cl2(g)��Kp��4.44��10��2��668K��KΪ��ƽ�ⳣ���������ܱ������У������������ѹ��Ϊ300kPaʱ������û�������ƽ����������
2-1 ��1��̼���������1�֣�
��2��COCl2��4NH3 �� NH2CONH2��2NH4Cl��1�֣�
��3��![]() ��CO��
��CO��![]() ��1�֣�
��1�֣�
��4��![]() ��NHCOCl
��NHCOCl ![]() ��N��C��O��2�֣�
��N��C��O��2�֣�
2-2 CCl4��H2SO4?SO3 ��COCl2��2ClSO3H����HCl��SO3����2�֣�
2-3 ��1��Cl3C��O��![]() ��O��CCl3��1�֣�
��O��CCl3��1�֣�
��2��BTC������Ϊ���ȶ��Ĺ������ʣ��������ˣ�1�֣���ʹ�ð�ȫ������Ⱦ��
2-4
COCl2��g��![]() CO��g����Cl2��g��
CO��g����Cl2��g��
��ʼ 1mol 0 0
ƽ�� 1��x x x
ƽ��ʱ�ܵ����ʵ���n���ܣ���1��x��1�֣�
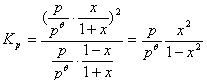 ��1�֣�
��1�֣�
x��0.121mol��1�֣�
![]() ��1�֣�
��1�֣�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com