
��ϸ��������ĩ���㷺Ӧ���ڴ��ģ���ɵ�·��������������ȡԭ��Ϊ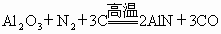 ���ڷ�Ӧ����ȫ����������Ʒ����������̿�����������ʣ�Ϊ�ⶨ�ò�Ʒ���йسɷֵĺ�������������������ʵ�飺
���ڷ�Ӧ����ȫ����������Ʒ����������̿�����������ʣ�Ϊ�ⶨ�ò�Ʒ���йسɷֵĺ�������������������ʵ�飺
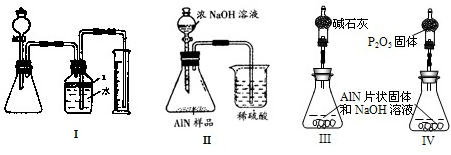
(1)��ȡ10.00g��Ʒ��������������NaOHŨ��Һ�й��Ȳ����ɣ�AlN��NaOH��Ӧ���� ���ų�����3.36L(��״��)��
���ų�����3.36L(��״��)��
��������Ӧ�Ļ�ѧ����ʽΪ_____________________��
�ڸ���Ʒ��AlN����������Ϊ______________��
(2)��ȡ10.00g��Ʒ���ڷ�Ӧ���У�ͨ��2.016gL(��״��) ���ڸ����³�ַ�Ӧ����������ܶ�Ϊ
���ڸ����³�ַ�Ӧ����������ܶ�Ϊ (������ɱ�״����AlN����
(������ɱ�״����AlN���� ��Ӧ)������Ʒ�к�����̿_________g��
��Ӧ)������Ʒ�к�����̿_________g��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� |
| 4100a |
| 22.4w |
| 4100a |
| 22.4w |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1����ȡ
��������Ӧ�Ļ�ѧ����ʽΪ_____________��?
�ڸ���Ʒ�е�AlN����������Ϊ___________��?
��2����ȡ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Al2O3��![]() 2AlN��3CO
2AlN��3CO
���ڷ�Ӧ����ȫ����������Ʒ����������̿�����������ʣ�Ϊ�ⶨ�ò�Ʒ���йسɷֵĺ�������������������ʵ�飺
��1����ȡ

��������Ӧ�Ļ�ѧ����ʽΪ___________________________________��
�ڸ���Ʒ�е�AlN����������Ϊ_______________��
����װ��B����ѡ����Լ���________________������ţ���
a.H2O b.ŨH2SO
������ʵ�鷽��������������������������������ϴ����˽�����������ҡ�������װ���е�һ�֣���ͨ����ڽ��У���ֻ����м��ֱ�Ҫ�����ݲⶨ���ɱȽ�ȷ��ȷ����Ʒ��AlN�������������Ϻ�����װ����___________������ţ���
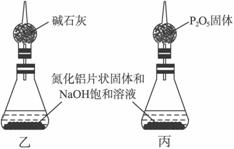
��2����ȡ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com