



 ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д� ��ĩ���ƾ�ϵ�д�
��ĩ���ƾ�ϵ�д� ���ɿ��ñ���ϵ�д�
���ɿ��ñ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ʵ�鷽�� | ʵ�鷽�� | �������� |
| A�� | �ζ��� | ����Ʒ���100 mL��Һ��ȡ10 mL��������ȣ��ñ�����ζ� | ������������ |
| B�� | ������ | ����Ʒ�����ᷴӦ�����ɵ�����ȫ������ʯ������ | ��ʯ������ |
| C�� | ������ | ��Ʒ������ƿ�У����ڵ�����ƽ�ϣ������������� | ��������� |
| D�� | ������ | ����Ʒ�����ᷴӦ������ͨ����ˮ����װ������ | ��ˮ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

| ʵ����� | ʵ������ | ��ѧ����ʽ |
| �� | �������������Һ���dz��ɫ | |
| �� | ����dz��ɫ���� | Na2S��Cl2��2NaCl��S�� |
| �� | | 2Na��2H2O��2NaOH��H2�� |
| �� | ���ҷ�Ӧ��Ѹ�ٲ�����ɫ���� | Mg��2HCl��MgCl2��H2�� |
| �� | ��Ӧ��ʮ�־��ң�������ɫ���� | 2Al��6HCl��2AlCl3��3H2�� |
| �� | ��ʼʱ���ɰ�ɫ��״�������̶�������ʧ | AlCl3��3NaOH��Al(OH)3����3NaCl Al(OH)3��NaOH��NaAlO2��2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
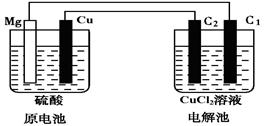
| A����þ�缫����������C1�� | B����C1������������þ�缫 |
| C����ͭ�缫����������C2�� | D����C2������������ͭ�缫 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com