
| A£®ŃÕÉ« |
| B£®ÖŹĮæ |
| C£®ĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ |
| D£®ĆÜ¶Č |

 Cu2++2NO2ӟ+2H2O
Cu2++2NO2”ü+2H2O N2O4(g)””¦¤H<0,ŌŚ·ŠĖ®ÖŠ,Ę½ŗā×óŅĘ,ŃÕÉ«¼ÓÉī”¢ĘųĢåĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ¼õŠ”,Ń¹ĒæŌö“ó,µ«ŹĒ»ģŗĻĘųĢåµÄÖŹĮæŗĶĆÜ¶Č²»±ä”£
N2O4(g)””¦¤H<0,ŌŚ·ŠĖ®ÖŠ,Ę½ŗā×óŅĘ,ŃÕÉ«¼ÓÉī”¢ĘųĢåĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ¼õŠ”,Ń¹ĒæŌö“ó,µ«ŹĒ»ģŗĻĘųĢåµÄÖŹĮæŗĶĆÜ¶Č²»±ä”£


| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā

| A£®¼×ŹĒÅØ°±Ė®£¬ŅŅŹĒÅØĮņĖį |
| B£®¼×ŹĒÅØŃĪĖį£¬ŅŅŹĒÅØ°±Ė® |
| C£®¼×ŹĒÅØ°±Ė®£¬ŅŅŹĒÅØŃĪĖį |
| D£®¼×ŹĒÅØĻõĖį£¬ŅŅŹĒÅØ°±Ė® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
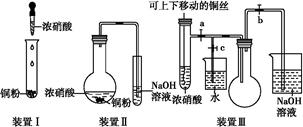
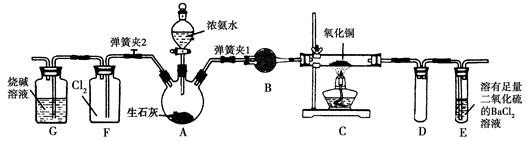
| | ŹµŃé×°ÖĆ | ŹµŃéŅ©Ę· | ÖʱøŌĄķ |
| ¼×Š”×é | A | ĒāŃõ»ÆøĘ”¢ĀČ»Æļ§ | ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ¢Ł |
| ŅŅŠ”×é | ¢Ś | ÅØ°±Ė®”¢ĒāŃõ»ÆÄĘ | ÓĆ»ÆŃ§Ę½ŗāŌĄķ·ÖĪöĒāŃõ»ÆÄʵÄ×÷ÓĆ£ŗ ¢Ū |
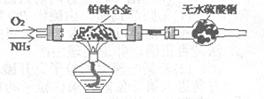
 3Cu+N2+3H2O£©£¬¶ųµŖĘųøśĆ¾ŌŚøßĪĀĻĀ·“Ó¦æɵƵ½µŖ»ÆĆ¾£¬µ«µŖ»ÆĆ¾ÓöĖ®Į¢¼“·“Ӧɜ³ÉMg£ØOH£©2ŗĶNH3”£ŅŅ×éĢį³öĮĖČēĻĀÖʱøµŖ»ÆĆ¾µÄŹµŃé·½°øŹ¾ŅāæņĶ¼£ØŹµŃéĒ°ĻµĶ³ÄŚæÕĘųŅŃÅųż£»Ķ¼ÖŠ¼żĶ·±ķŹ¾ĘųĢåµÄĮ÷Ļņ£©”£ÄćČĻĪŖ“Ė·½°øŹĒ·ńÕżČ·£¬²¢ĖµĆ÷ĄķÓÉ ”£
3Cu+N2+3H2O£©£¬¶ųµŖĘųøśĆ¾ŌŚøßĪĀĻĀ·“Ó¦æɵƵ½µŖ»ÆĆ¾£¬µ«µŖ»ÆĆ¾ÓöĖ®Į¢¼“·“Ӧɜ³ÉMg£ØOH£©2ŗĶNH3”£ŅŅ×éĢį³öĮĖČēĻĀÖʱøµŖ»ÆĆ¾µÄŹµŃé·½°øŹ¾ŅāæņĶ¼£ØŹµŃéĒ°ĻµĶ³ÄŚæÕĘųŅŃÅųż£»Ķ¼ÖŠ¼żĶ·±ķŹ¾ĘųĢåµÄĮ÷Ļņ£©”£ÄćČĻĪŖ“Ė·½°øŹĒ·ńÕżČ·£¬²¢ĖµĆ÷ĄķÓÉ ”£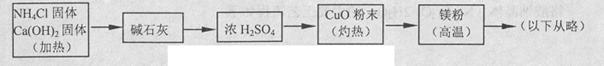
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ŹµŃéŹŅÖĘĒāĘų£¬ĪŖĮĖ¼Óæģ·“Ó¦ĖŁĀŹ£¬æÉĻņĻ”H2SO4ÖŠµĪ¼ÓÉŁĮæCu£ØNO3£©2ČÜŅŗ |
| B£®ĪŖ“¦Ąķ¹ųĀÆĖ®¹øÖŠµÄCaSO4£¬æÉĻČÓƱ„ŗĶNa2CO3ČÜŅŗ½žÅŻ£¬ŌŁ¼ÓČėŃĪĖįČܽā |
C£®N2£Øg£©£«3H2£Øg£© 2NH3£Øg£© ¦¤H£¼0£¬ĘäĖūĢõ¼ž²»±äŹ±ÉżøßĪĀ¶Č£¬Ę½ŗāŹ±ĒāĘų×Ŗ»ÆĀŹŌö“ó 2NH3£Øg£© ¦¤H£¼0£¬ĘäĖūĢõ¼ž²»±äŹ±ÉżøßĪĀ¶Č£¬Ę½ŗāŹ±ĒāĘų×Ŗ»ÆĀŹŌö“ó |
| D£®ĪüČČ·“Ó¦”°TiO2£Øs£©£«2Cl2£Øg£©£½TiCl4£Øg£©£«O2£Øg£©”±ŌŚŅ»¶ØĢõ¼žĻĀæÉ×Ō·¢½ųŠŠ£¬ŌņøĆ·“Ó¦µÄ¦¤S£¼0 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com