
 A��B��C��D��E��FΪԪ�����ڱ�ǰ�����ڵ�Ԫ�أ�ԭ��������������AԪ�صĵ����ǿ�������Ҫ�ɷ֣�Bԭ�Ӻ���p�������1�ԳɶԵ��ӣ�DԪ�صļ۵������������������һ�룬C��Bͬ���壬A��Fͬ���壬D��Eͬ�壮�ش��������⣺
A��B��C��D��E��FΪԪ�����ڱ�ǰ�����ڵ�Ԫ�أ�ԭ��������������AԪ�صĵ����ǿ�������Ҫ�ɷ֣�Bԭ�Ӻ���p�������1�ԳɶԵ��ӣ�DԪ�صļ۵������������������һ�룬C��Bͬ���壬A��Fͬ���壬D��Eͬ�壮�ش��������⣺| 6-2��2 |
| 2 |
| 6-2��3 |
| 2 |
| 5+1-2��3 |
| 2 |
| 6+2-2��3 |
| 2 |
| 6+2-2��4 |
| 2 |
| 1 |
| 8 |
| 1 |
| 4 |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��NO2 |
| B��Cl2 |
| C��HCl |
| D��NH3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B | C | D | |
| ǿ����� | H2SO4 | NaCl | HClO | HNO3 |
| ������� | HF | NH3 | CaCO3 | H2CO3 |
| �ǵ���� | Cl2 | CH3COOH | C2H5OH | SO2 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����£���10mL 0.1mol?L-1��H2C2O4��Һ����μ���0.1mol?L-1 KOH��Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ�������
�����£���10mL 0.1mol?L-1��H2C2O4��Һ����μ���0.1mol?L-1 KOH��Һ�����õζ�������ͼ��ʾ������˵����ȷ���ǣ�������| A��KHC2O4��Һ�������� |
| B��B��ʱ��c��K+����c��HC2O4-����c��C2O42-����c��H+����c��OH-�� |
| C��C��ʱ��c��K+����c��HC2O4-��+c��C2O42-��+c��H2C2O4�� |
| D��D��ʱ��c��H+��+c��HC2O4-��+c��H2C2O4��=c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� | �� | �� | |
| �� | �� | ||
| �� |
| A���ҡ��졢������Ԫ���γɵĻ�����ֻ�����ۼ� |
| B��ԭ�Ӱ뾶���ף��ң��� |
| C������������Ӧˮ��������ԣ��죾������ |
| D�����뼺��ԭ���������8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��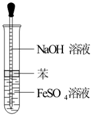 �Ʊ�Fe��OH��2 |
B�� ��֤���뱽����ȡ����Ӧ |
C�� �ⶨ�к��� |
D�� �Ʊ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��CH3CH2CHBrCH2Br |
| B��CH3CH��CH2Br��2 |
| C��CH3CHBrCHBrCH3 |
| D����CH3��2CBrCH2Br |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��6��3��2 |
| B��1��2��3 |
| C��3��2��1 |
| D��4��3��2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com