
��ˮռ�����ܴ�ˮ����97%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
��1����ҵ�����õ�ⱥ��ʳ��ˮ���Ƶ���Ҫ������Ʒ���ֳ�Ϊ���ȼҵ����Ҳ���Ե�������Ȼ����Ƶ��ƺ��������÷�Ӧ����ʽΪ________________________��
��2�����������������һ�������ȼҵ��Ʒ���Ȼ���ѭ������������������������ն�������ķ������÷������������£�
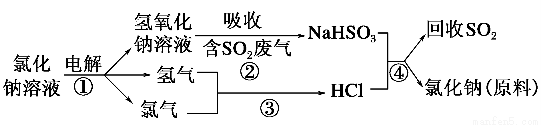
��д����Ӧ�Ļ�ѧ����ʽ��
��__________����__________����__________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014����л�ѧ���ִ���ѵ���� ר��14���ʽṹ������ѡ����ϰ���������棩 ���ͣ������
�±�Ϊ���ڱ���һ���������еı�Ŵ�����Ӧ��Ԫ�ء�

����ա�
��1��д���ϱ���Ԫ��I�Ļ�̬ԭ�ӵĵ����Ų�ʽ�ͼ۲�����Ų�ͼ��________________________________________________________________________��
Ԫ��C��D��E��F�ĵ�һ�������ɴ�С��˳����________����Ԫ�ط��ű�ʾ����
��2��Ԫ��A�ֱ���C��D��E�γ����ij�����������Ӽס��Һͱ��������й���������ȷ����________��
A���ס��Һͱ����ӵĿռ乹�ͷֱ�Ϊ���������Ρ������Ρ�V��
B���ס��Һͱ�������������ԭ�Ӿ���ȡsp3���ӻ���ʽ
C�����ַ����м����ɴ�С��˳���DZ����ң���
D���ס��Һͱ����Ӿ�Ϊ�ɼ��Լ����ɵļ��Է���
��3����Ԫ��J��C��E���һ�ֻ�ѧʽΪJ��CE��5����λ�������������ʳ����³�Һ̬���۵�Ϊ��20.5 �棬�е�Ϊ103 �棬�����ڷǼ����ܼ����ݴ˿��жϣ�
���û�����ľ�������Ϊ________��
���û�����ľ����д��ڵ���������________��
A�����Ӽ� B�����Լ�
C���Ǽ��Լ� D�����»���
E����� F�����
�����ݹ��ۼ����ۺ͵ȵ��������۷�����CE��������������������Ŀ��Ϊ________��
��4���ڲⶨA��F�γɵĻ��������Է�������ʱ��ʵ���õ�ֵһ���������ֵ����Ҫԭ����________________________________________________��
��5��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ�����������������Ԫ��G��Ԫ��B��ԭ����_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰����1-2-3Ԫ�����ڱ�Ԫ��������Ӧ����ϰ���������棩 ���ͣ������
�������߷ֱ��ʾԪ�ص�ij��������˵�����Ĺ�ϵ��ZΪ�˵������YΪԪ�ص��й����ʣ���
��1����������Ԫ���й�������������߱��������Ӧ�Ŀո��У�
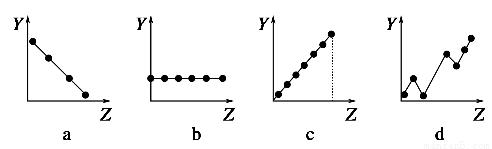
������A��Ԫ�صļ۵�����________��
����������Ԫ�ص�����ϼ�________��
��F����Na����Mg2����Al3�������Ӱ뾶________��
��2��Ԫ��X��Y��Z��M��N��Ϊ����������Ԫ�أ���ԭ����������������֪Yԭ������������������������֮��Ϊ3��4��MԪ��ԭ�ӵ���������������Ӳ���֮��Ϊ4��3��N����Z����X���İ뾶��С��������XN������Ϊ���塣�ݴ˻ش�
��XΪ________�����ƣ���YΪ________��Ԫ�ط��ţ���Zԭ�ӽṹʾ��ͼΪ________��
��N������������ˮ����Ļ�ѧʽΪ__________��
����ҵ����ȡ����M�Ļ�ѧ����ʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-2��Դ�ۺ����á�����������ϰ���������棩 ���ͣ�ʵ����
ijͬѧ�������ͼ��ʾװ�ý���ʯ�ͷ����ʵ�顣
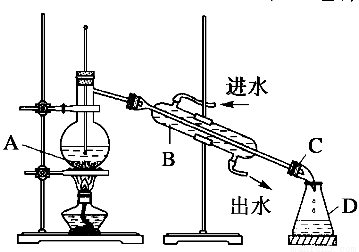
��1��ָ��ʵ��װ��������A��B��C��D�����ƣ�
A________��B________��C________��D________��
��2��ָ����ͬѧ����Ƶ�ʵ��װ���д��ڵĴ������������
������_______________________________________��
��_____________________________________________��
��������_______________________________________��
��_____________________________________________��
��3��ʵ��װ�ø������������Լ��ķ�����______________________________��
��4��������ƿ�з��뼸Ƭ���Ƭ��������__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-2��Դ�ۺ����á�����������ϰ���������棩 ���ͣ�ѡ����
������ɫ��ѧ�������У�����״̬�Ƿ�Ӧ���е�ԭ��ȫ��ת��Ϊ���ƵõIJ����ԭ��������Ϊ100%�����з�Ӧ������������ԭ�Ӿ�������ԭ����ǣ� ��
���û���Ӧ�������Ϸ�Ӧ�����ֽⷴӦ����ȡ����Ӧ�����ӳɷ�Ӧ������ȥ��Ӧ�����Ӿ۷�Ӧ�������۷�Ӧ
A���٢ڢ� B���ڢݢ� C���ߢ� D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-1-2��ˮ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
����˵����ȷ���ǣ� ��
A����ˮ�к�����ߵ�Ԫ������
B����ˮ�к�����ߵ��������Ȼ���
C���ӵ�ʳ����Һ��������Һ����ɫ
D���ӵ�ʳ���еĵ��Ǻ���Ԫ�صĻ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-1-2��ˮ��Դ�Ŀ���������ϰ���������棩 ���ͣ�ѡ����
���й��ں�ˮ֪ʶ�������в���ȷ���ǣ� ��
A����ˮ��Դ�����ð�����ˮˮ��Դ�����úͺ�ˮ��ѧ��Դ������
B����ˮ�ȿ���ɬ������ֱ������
C����ˮ��ijЩ����Ԫ���ܴ����������̶�ȴ�ܵ�
D����ˮ����Ӧͬ������������Դ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 4-1-1��������Ŀ���������ϰ���������棩 ���ͣ�ѡ����
��������ܶ���С�Ľ������������IJ�ͬ������﮿����ͷų�����ĵ��ӣ��ʳ�������������ܵ�ء���֪﮵Ľ����Խ����ƺ�þ֮�䣬��ұ�������Ӧ���õķ����ǣ� ��
A����ⷨ B���Ȼ�ԭ��
C���ȷֽⷨ D�����ȷ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014���һ��ѧ�˽̰���� 3-3-2������ϰ���������棩 ���ͣ�ѡ����
���з�Ӧ�У�����ȡ����Ӧ���ǣ� ��
��CH3CH=CH2��Br2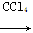 CH3CHBrCH2Br
CH3CHBrCH2Br
��CH3CH2OH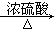 CH2=CH2����H2O
CH2=CH2����H2O
��CH3COOH��CH3CH2OH CH3COOCH2CH3��H2O
CH3COOCH2CH3��H2O
��C6H6��HNO3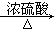 C6H5NO2��H2O
C6H5NO2��H2O
A���٢� B���ۢ� C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com