
ij��ɫ����Һ�п��ܺ���Ag+��Fe3+��K+��Ba2+��NH4+�������ӡ�ijͬѧ��������ʵ�飺
I�����������ϡ���ᣬ�а�ɫ�������ɡ�
II�����ˣ�ȡ������Һ�������м��������ϡ���ᣬ���а�ɫ�������ɡ�
III����ȡ��������II�е���Һ������NaOH��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��1������Һ��һ�����е�������__________��һ�������е�������___________��
��2������III�в�����������ӷ���ʽΪ__________________��
��1��Ag+��Ba2+��NH4+ Fe3+��2��NH4++OH�� NH3��+H2O
NH3��+H2O
���������������������֪����ҺΪ��ɫ����Fe3+ ��ˮ��Һ�����ػ�ɫ����һ��û�� Fe3+�����ݢ�֪�����������ϡ���ᣬ�а�ɫ�������ɣ���Һ��һ���� Ag+�����ݢ�֪�����������ϡ���ᣬ���а�ɫ�������ɣ���Һ�����ԣ���Һ��һ���� Ba2+�����ݢ�֪������NaOH��Һ����Һ�ʼ��ԣ����ȣ��ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����壬��Һ��һ���� NH4+��������������֪����1������Һ��һ�����е������� Ag+��Ba2+��NH4+�� һ�������е�������Fe3+���� 3����κ��������Ʒ�Ӧ���ӷ���ʽΪNH4++OH�� NH3��+H2O ��
NH3��+H2O ��
���㣺�������Ӽ���������ƶϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ͬѧ����Ϥ�����ʣ�
��O2���ڽ��ʯ����NaBr����H2SO4����Na2CO3
��NH4Cl����NaHSO4����Ne����Na2O2����NaOH
(1)��Щ�����У�ֻ���й��ۼ�����________��ֻ�������Ӽ�����________���Ⱥ��й��ۼ��ֺ������Ӽ�����________�������ڻ�ѧ������________��
(2)���ڹ��ۻ��������________���������ӻ��������________��
(3)��NaHSO4����ˮ���ƻ���NaHSO4�е�________��д������뷽��ʽ_________________________��
NaHSO4������״̬�µ��룬�ƻ���________��д������뷽��ʽ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ú��ʯ��Ԥ������SiO2��63%����Al2O3��25%����Fe2O3��5%����������þ�Ļ�����ȣ�һ���ۺ����ù���������£�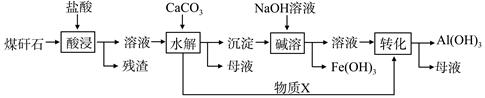
��1�����������������Ҫ��Ӧ�����ӷ���ʽΪ_____________��_________________��
��2���������ʱ�������ʵ�Ӱ�����ؿ�����_____________��___________����д��������
��3������X�Ļ�ѧʽΪ___________�������ܡ�ʱ��Ӧ�����ӷ���ʽΪ____________��
��4����֪Fe3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ2.1��3.2��Al3+��ʼ�����ͳ�����ȫ��pH�ֱ�Ϊ4.1��5.4��Ϊ�˻�ò�ƷAl��OH�� 3����ú��ʯ�������ȡҺ��ʼ����ֻ��CaCO3һ���Լ�����������������____________________��
��5����ú��ʯΪԭ�ϻ����Կ���������Ʒ��������ú��ʯ�������ȡҺ������������AlCl3��Һ�в���ͨ��HCl���壬����������AlCl3��6H2O���壬��ϻ�ѧƽ���ƶ�ԭ���������������ԭ��_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ӷ�Ӧ����ѧ��ѧ����Ҫ�ķ�Ӧ���ͣ��ش��������⣺
(1)�ڷ������ӷ�Ӧ�ķ�Ӧ����������У�һ������________(����)��
�ٵ��� �������� �۵���� ���� �ݻ�����
(2)һ����ɫ����Һ�У����ܺ����������ӣ� ��
�� ��I-��Cl-��ȡ����Һ��������ʵ�飺
��I-��Cl-��ȡ����Һ��������ʵ�飺
�ٽ���Һ������ɫʯ����ֽ�ϣ��ʺ�ɫ��
�ڽ�������ҺŨ�������ͭƬ�����ᣬ����ɫ����������������ͨ������������ɺ���ɫ��
��ȡ������Һ����BaCl2��Һ���������ɫ������
��ȡʵ����еij�����Һ������AgNO3��Һ������������ϡ����İ�ɫ������
����ȡ������Һ������NaOH��Һ���а�ɫ�������ɣ���NaOH����ʱ�����в��ְ�ɫ�����ܽ⡣
�������������жϣ�ԭ��Һ�п϶������ڵ�������_______���϶����ڵ�������_______�����������жϵ�������_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�������ȣ�ClO2����һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ��������������Cl2��Ȳ��������������DZ��Σ�����л��ȴ���Ʊ�ClO2���������ַ�����
����һ��2 NaClO3 + 4 HCl��2 ClO2��+ Cl2��+ 2 NaCl + 2 H2O
��������2 NaClO3 + H2O2 + H2SO4��2 ClO2��+ O2��+2 Na2SO4 + 2 H2O
��1������һ�����ӷ���ʽΪ ��
��2���������б������������� ������Ӧ����0.1mol����ת�ƣ��������ClO2�����ڱ�״���µ����Ϊ L��
��3���뷽��һ�Ƚϣ��÷������Ʊ���ClO2���ʺ���������ˮ������������Ҫԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������A��B��C��D��E�����ǵ������ӿ�����Na����NH4+��Cu2����Ba2����Al3����Ag����Fe3���������ӿ�����Cl����NO3����SO42����CO32������֪��
�������ξ�����ˮ��ˮ��Һ��Ϊ��ɫ��
��D����ɫ��Ӧ�ʻ�ɫ��
��A����Һ�����ԣ�B��C��E����Һ�����ԣ�D����Һ�ʼ��ԡ�
�������������ε���Һ�зֱ����Ba(NO3)2��Һ��ֻ��A��C����Һ������������
�������������ε���Һ�У��ֱ���백ˮ��E��C����Һ�����ɳ����������Ӱ�ˮ��C�г�����ʧ��
�ް�A����Һ�ֱ���뵽B��C��E����Һ�У��������ɲ�����ϡ����ij�����
��ش��������⡣
(1)�������У�һ��û�е���������____________��������������ͬ�������εĻ�ѧʽ��________________��
(2)D�Ļ�ѧʽΪ______________��D��Һ�Լ��Ե�ԭ����(�����ӷ���ʽ��ʾ)__________________________________________��
(3)A��C����Һ��Ӧ�����ӷ���ʽ��_________________________��E�Ͱ�ˮ��Ӧ�����ӷ���ʽ��____________________��
(4)��Ҫ����B�������������ӣ���ȷ��ʵ�鷽����________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ճ�����ˮ��NO3һ�ĺ�����ˮ�������������Ҫ��֮һ���ﵽһ��Ũ��ʱ������ཡ������Σ����Ϊ�˽�������ˮ��NO3һ��Ũ�ȣ�ij��ȤС��������·�����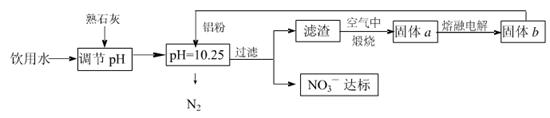
��ش��������⣺
��1���÷����ڵ���pHʱ����pH������С������� �������ʽ��͡�
��2����֪���˺�õ�����Һ�м���������Ԫ�ء�������Һ�����ۺ�NO3һ��Ӧ�����ӷ���ʽΪ ��
�������ڿ��������չ������漰����ط�Ӧ����ʽΪ
��3����H2����ԭ��Ҳ�ɽ�������ˮ��NO3����Ũ�ȣ���֪��Ӧ�еĻ�ԭ���������������ɲ������ѭ���������ԭ�������ӷ���ʽΪ ��
��4������ˮ�е�NO3�� ��Ҫ������NH4������֪�����������£�NH4�� ����������Ӧ��������NO3�� ��������Ӧ�������仯ʾ��ͼ���£�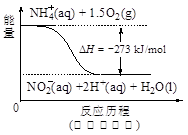

��д��1 mol NH4�� (aq)ȫ��������NO3�� (aq)���Ȼ�ѧ����ʽ�� ��
��5������a���ڵ��ʱ������ӦʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.��4�֣�ʵ������һƿ�������Һ��ʵ����Աȷ�����п��ܺ���NH4+��K+��Na+��Mg2+��Ba2+��Al3+��Fe3+��Cl-��I-��NO3-��CO32-��SO42-��ȡ����Һ��������ʵ�飺
��ȡpH��ֽ���飬������Һ��ǿ���ԡ�
��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ��
����ȡ������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ������������
��ȡ��������������Һ��Na2CO3��Һ���а�ɫ�������ɡ�
�ݽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
����ڵ�ˮ���м���HNO3�ữ��AgNO3��Һ�а�ɫ������
��������ʵ����ʵȷ���������жϸ���Һ��
��1���϶����ڵ������� ��
��2������ȷ���Ƿ���ڵ������� ��
��. ��6�֣���������(SeO2)��һ�����������䱻��ԭ��ĵ��������ܳ�Ϊ������Ⱦ�ͨ����ŨHNO3��ŨH2SO4��Ӧ����SeO2�Ի���Se�����������գ�
��1��Se��ŨHNO3��Ӧ�Ļ�ԭ����ΪNO��NO2����NO��NO2�����ʵ���֮��Ϊ1��1��д��Se��ŨHNO3�ķ�Ӧ����ʽ ��
��2����֪��Se+2H2SO4(Ũ)��2SO2��+SeO2+2H2O��2SO2+SeO2+2H2O��Se+2SO42-+4H+
SeO2��H2SO4(Ũ)��SO2����������ǿ������˳���� ��
��3�����յõ���SeO2�ĺ���������ͨ������ķ����ⶨ��
��SeO2+ 4KI+ 4HNO3��Se+2I2+ 4KNO3+2H2O ��I2+2Na2S2O3��Na2S4O6+2NaI
ʵ���У�ȷ����SeO2��Ʒ0.1500g��������0.2000 mol/L��Na2S2O3��Һ25.00 mL�����ⶨ����Ʒ��SeO2����������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
д�����з�Ӧ�����ӷ���ʽ,����Ӧ�Ļ�ѧ����ʽ��ÿ��2��7С�14�֣�
��1��ʯ��ʯ����ϡ���� ��
��2��ϡ����������������Һ�ķ�Ӧ ��
��3��̼��������Һ�����ᷴӦ ��
��4��������ͭ��ϡ���ᷴӦ ��
��5��2H++ CO32-��CO2��+H2O ��
��6��Cu��2Ag����Cu2����2Ag ��
��7��CO2+2OH-��CO32-+H2O ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com