
”¾ĢāÄæ”æŃĒĻõõ£ĀČNOClæÉÓĆÓŚŗĻ³ÉĒå½ą¼ĮµČ”£ĖüæÉÓĆCl2ÓėNOŌŚ³£ĪĀ³£Ń¹ĻĀŗĻ³É£ŗĖüµÄČŪµćĪŖ-64.5”ę£¬·ŠµćĪŖ-5.5”ę£¬³£ĪĀĻĀŹĒ»ĘÉ«µÄÓŠ¶¾ĘųĢ壬ÓöĖ®Ņ×Ė®½ā”£ Ēė°“ŅŖĒó»Ų“šĻĀĮŠĻą¹ŲĪŹĢā£ŗ
(1)¹żĮæĢśŠ¼ŗĶĻ”ĻõĖį³ä·Ö·“Ó¦ÖʱøNOµÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ______________________”£
(2)ÖʱøNOClµÄ×°ÖĆČēĻĀĶ¼ĖłŹ¾£¬Į¬½ÓĖ³ŠņĪŖ:a”ś_________________________(°“ĘųĮ÷×Ō×óĻņÓŅ·½Ļņ£¬ÓĆŠ”Š“×ÖÄø±ķŹ¾)”£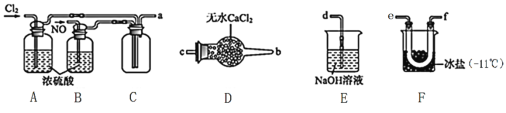
¢Ł×°ÖĆAŗĶB×÷ÓĆŹĒ¢ŁøÉŌļNOŗĶCl2£¬¢Ś___________________________________________”£
¢Ś×°ÖĆDµÄ×÷ÓĆŹĒ______________________________________”£
¢Ū×°ÖĆEÖŠNOCl·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ________________”£
(3)¹¤ŅµÉś²ś¹ż³ĢÖŠNOĪ²Ęų“¦Ąķ·½·ØÓŠ¶ąÖÖ£¬ĘäÖŠ¼ä½Óµē»Æѧ·Ø£¬ĘäŌĄķČēĶ¼ĖłŹ¾£ŗ

øĆ¹ż³ĢÖŠŅõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ£ŗ__________________________________________”£
”¾“š°ø”æ3Fe+8H++2NO3”Ŗ=3Fe2++2NO”ü+4H2O e”śf”śc”śb”śd»ņf”śe”śc”śb”śd ¹Ū²ģĘųÅŻµ÷½ŚĘųĢåĮ÷ĖŁ ·ĄÖ¹EÖŠĖ®ÕōĘų½ųČėF£¬ŅżĘšNOClµÄĖ®½ā NOCl+2NaOH=NaCl+NaNO2+H2O 2HSO3”Ŗ+2e”Ŗ+2H+= S2O42”Ŗ+ 2H2O
”¾½āĪö”æ
£Ø1£©¹żĮæĢśŠ¼ŗĶĻ”ĻõĖį·“Ӧɜ³ÉĻõĖįŃĒĢś”¢Ņ»Ńõ»ÆµŖŗĶĖ®£»
£Ø2£©½«ĀČĘųŗĶNOøÉŌļŗóŌŚ×°ÖĆFÖŠ·¢Éś·“Ó¦£¬ŌŚ±łŃĪÖŠĄäÄżŹÕ¼ÆNOCl£¬ĀČĘų”¢NOŅŌ¼°NOCl¾ł²»ÄÜÅŷŵ½æÕĘųÖŠ£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗĪüŹÕ£¬×¢ŅāNOClÓöĖ®Ņ×Ė®½ā”£
£Ø3£©Óɼä½Óµē»Æѧ·ØŹ¾ŅāĶ¼æÉÖŖ£¬Ņõ¼«ÉĻHSO3”Ŗ·ÅµēÉś³ÉS2O42”Ŗ£¬S2O42”ŖŌŚĪüŹÕĖžÖŠ½«NO»¹ŌĪŖN2”£
£Ø1£©¹żĮæĢśŠ¼ŗĶĻ”ĻõĖį·“Ӧɜ³ÉĻõĖįŃĒĢś”¢Ņ»Ńõ»ÆµŖŗĶĖ®£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ3Fe+8H++2NO3”Ŗ=3Fe2++2NO”ü+4H2O£¬¹Ź“š°øĪŖ£ŗ3Fe+8H++2NO3”Ŗ=3Fe2++2NO”ü+4H2O£»
£Ø2£©½«ĀČĘųŗĶNOøÉŌļŗóŌŚ×°ÖĆFÖŠ·¢Éś·“Ó¦£¬ŌŚ±łŃĪÖŠĄäÄżŹÕ¼ÆNOCl£¬ĀČĘų”¢NOŅŌ¼°NOCl¾ł²»ÄÜÅŷŵ½æÕĘųÖŠ£¬ÓĆĒāŃõ»ÆÄĘČÜŅŗĪüŹÕ£¬NOClÓöĖ®Ņ×Ė®½ā£¬¹ŹŌŚŹÕ¼Æ×°ÖĆŗĶĪ²Ęų“¦Ąķ×°ÖĆÖ®¼äŠč¼ÓŅ»øöøÉŌļ×°ÖĆ£¬¹Ź“š°øĪŖ£ŗe”śf£Ø»ņf”śe£©”śc”śb”śd£»
¢Ł×°ÖĆAŗĶB×÷ÓĆŹĒ³żøÉŌļNO”¢Cl2Ķā£¬ĮķŅ»øö×÷ÓĆŹĒĶعż¹Ū²ģĘųÅŻµ÷½ŚĘųĢåµÄĮ÷ĖŁæŲÖĘ·“Ó¦µÄ·¢Éś£¬¹Ź“š°øĪŖ£ŗ¹Ū²ģĘųÅŻµ÷½ŚĘųĢåĮ÷ĖŁ£»
¢ŚNOClÓöĖ®Ņ×Ė®½ā£¬×°ÖĆDÖŠĀČ»ÆøĘ×öøÉŌļ¼Į£¬ĪüŹÕĖ®ÕōĘų£¬·ĄÖ¹EÖŠĖ®ÕōĘų½ųČė·“Ó¦Ę÷FÖŠ£¬ŅżĘšNOClµÄĖ®½ā£¬¹Ź“š°øĪŖ£ŗ·ĄÖ¹EÖŠĖ®ÕōĘų½ųČėF£¬ŅżĘšNOClµÄĖ®½ā£»
¢Ū×°ÖĆEµÄÄæµÄĪüŹÕĪ²Ęų£¬·ĄÖ¹ĪŪČ¾»·¾³£¬NOClÓėĒāŃõ»ÆÄĘČÜŅŗ·“Ӧɜ³ÉNaCl”¢NaNO2ŗĶH2O£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖNOCl+2NaOH=NaCl+NaNO2+H2O£¬¹Ź“š°øĪŖ£ŗNOCl+2NaOH=NaCl+NaNO2+H2O£»
£Ø3£©Óɼä½Óµē»Æѧ·ØŹ¾ŅāĶ¼æÉÖŖ£¬Ņõ¼«ÉĻHSO3”Ŗ·ÅµēÉś³ÉS2O42”Ŗ£¬S2O42”ŖŌŚĪüŹÕĖžÖŠ½«NO»¹ŌĪŖN2£¬Ņõ¼«µÄµē¼«·“Ó¦Ź½ĪŖ2HSO3”Ŗ+2e”Ŗ+2H+= S2O42”Ŗ+ 2H2O£¬¹Ź“š°øĪŖ£ŗ2HSO3”Ŗ+2e”Ŗ+2H+= S2O42”Ŗ+ 2H2O”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ×ŌĄ“Ė®æÉÓĆĀČĘųĻū¶¾£¬Ä³Ń§ÉśÓĆÕāÖÖ×ŌĄ“Ė®ÅäÖĘĻĀĮŠĪļÖŹµÄČÜŅŗ£¬²»»į²śÉśĆ÷ĻŌŅ©Ę·±äÖŹĪŹĢāµÄŹĒ( )
A. AlCl3B. FeCl2C. AgNO3D. Na2CO3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æHg ŹĒĖ®ĢåĪŪČ¾µÄÖŲ½šŹōŌŖĖŲÖ®Ņ»”£Ė®ČÜŅŗÖŠµÄ¶ž¼Ū¹ÆµÄÖ÷ŅŖ“ęŌŚŠĪĢ¬ÓėCl-”¢OH-µÄÅØ¶Č¹ŲĻµČēĶ¼ĖłŹ¾[Ķ¼ÖŠÉę¼°µÄĪļÖŹ»ņĮ£×ÓÖ»ÓŠHg(OH)2 ĪŖÄŃČÜĪļ£¬Į£×ÓÅضČŗÜŠ”Ź±³£ÓĆøŗ¶ŌŹż±ķŹ¾£¬ČēpH=-lgc(H+)£¬pCl=-1gc(Cl-)]:

ĻĀĮŠĖµ·ØÖŠ“ķĪóµÄŹĒ
A. Hg(NO3)2¹ĢĢåČÜÓŚĖ®Ķس£»į³öĻÖ»ė×Ē
B. ŗ£Ė®Cl-µÄÅØ¶Č“óÓŚ0.1mol/L£¬ŌņĘäÖŠ¹ÆŌŖĖŲµÄÖ÷ŅŖ“ęŌŚŠĪĢ¬ŹĒHg(OH)2
C. ÉŁĮæHg(NO3)2ČÜÓŚ0.001moL/LŃĪĖįŗóµĆµ½³ĪĒåĶøĆ÷ČÜŅŗ
D. ŅŃÖŖKsp(HgS)=1.6”Į10-52£¬µ±c(S2-)=1”Į10-5mo/L Ź±£¬c(Hg2+)=1.6”Į10-47mo/L
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æt”ꏱ£¬ŌŚŅ»øöĢå»żĪŖ2LĆܱÕČŻĘ÷ÖŠ¼ÓČė·“Ó¦ĪļA”¢B£¬·¢ÉśČēĻĀ·“Ó¦£ŗA(s)+2B(g)![]() 3C(g)”£·“Ó¦¹ż³ĢÖŠµÄ²æ·ÖŹż¾ŻČēĻĀ±ķĖłŹ¾£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
3C(g)”£·“Ó¦¹ż³ĢÖŠµÄ²æ·ÖŹż¾ŻČēĻĀ±ķĖłŹ¾£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
ĪļÖŹ | ĘšŹ¼ | 2·ÖÖÓ | 4·ÖÖÓ | 6·ÖÖÓ |
A | 2 mol | 1.2 mol | ||
B | 6 mol | 3.0 mol | ||
C | 0 mol | x mol | 4.5 mol |
A. Ē°2·ÖÖÓÄŚ£¬AµÄ·“Ó¦ĖŁĀŹĪŖ0.2molL-1min-1
B. ±ķÖŠxµÄÖµĪŖ3.6
C. 4·ÖÖÓŹ±£¬·“Ó¦“ļµ½Ę½ŗāדĢ¬£¬“ĖŹ±Õż”¢Äę·“Ó¦µÄĖŁĀŹ¶¼ĪŖ0
D. ÉżøßĪĀ¶Č£¬Õż”¢Äę·“Ó¦µÄĖŁĀŹ¶¼»įŌö“ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĪŖĮĖĢ½¾æij¹ĢĢå»ÆŗĻĪļX£Øŗ¬ÓŠ3ÖÖŌŖĖŲ£©µÄ×é³ÉŗĶŠŌÖŹ£¬Éč¼Ę²¢Ķź³ÉČēĻĀŹµŃ飬Ēė»Ų“š£ŗ

ŅŃÖŖ£ŗĘųĢå¼×ÖŠŗ¬ÓŠĮ½ÖÖĘųĢå£Ø×é³ÉŌŖĖŲĻąĶ¬£©£¬ĒŅĪŽÉ«ĪŽĪ¶”£
£Ø1£©¹ĢĢåXÖŠŗ¬ÓŠŃõ”¢_________ŗĶ__________ČżÖÖŌŖĖŲ£ØŠ“ŌŖĖŲ·ūŗÅ£©
£Ø2£©Š“³öXøō¾ųæÕĘų·Ö½āµÄ»Æѧ·½³ĢŹ½___________________________________________£»
£Ø3£©¼ģŃéČÜŅŗAÖŠ×īÖ÷ŅŖ½šŹōŃōĄė×ӵķ½·ØŹĒ____________________£»
£Ø4£©ÓɳĮµķ×Ŗ»ÆĪŖŗģ×ŲÉ«¹ĢĢåµÄŅ»ĻµĮŠ²Ł×÷°üĄØ£ŗ¹żĀĖ”¢_______”¢×ĘÉÕ”¢________ŗĶ³ĘĮ攣
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĄ±½·ĖŲŹĒĄ±½·µÄ»īŠŌ³É·Ö£¬æÉŅŌŌ¤·ĄŠÄŌą²”£¬Ņ²ÄÜ»ŗ½ā¼”Čā¹Ų½ŚĢŪĶ“£®Ą±½·ĖŲÖŠõ„Ąą»ÆŗĻĪļµÄ½į¹¹ČēĶ¼(RĪŖĢž»ł)£®ĘäÖŠŅ»ÖÖĄ±½·ĖŲõ„Ąą»ÆŗĻĪļJµÄŗĻ³ÉĀ·ĻßČēĻĀ£ŗ


ŅŃÖŖ£ŗ
¢ŁA”¢BŗĶEĪŖĶ¬ĻµĪļ£¬ĘäÖŠBµÄĻą¶Ō·Ö×ÓÖŹĮæĪŖ44£¬AŗĶBŗĖ“Ź²ÕńĒāĘ×ĻŌŹ¾¶¼ÓŠĮ½×é·å£»
¢Ś»ÆŗĻĪļJµÄ·Ö×ÓŹ½ĪŖC15H22O4£»
¢ŪR1CHO+R2CH2CHO![]()
![]() +H2O
+H2O
»Ų“šĻĀĮŠĪŹĢā£ŗ
(1)GĖłŗ¬¹ŁÄÜĶŵÄĆū³ĘĪŖ_____________________”£
(2)ÓÉAŗĶBÉś³ÉCµÄ»Æѧ·½³ĢŹ½ĪŖ_______________________________________”£
(3)ÓÉCÉś³ÉDµÄ·“Ó¦ĄąŠĶĪŖ_____________£¬DµÄ»ÆѧĆū³ĘĪŖ_____________________”£
(4)ÓÉHÉś³ÉIµÄ»Æѧ·½³ĢŹ½ĪŖ_____________________”£
(5)JµÄ½į¹¹¼ņŹ½ĪŖ_____________________”£
(6)GµÄĶ¬·ÖŅģ¹¹ĢåÖŠ£¬±½»·ÉĻµÄŅ»ĀČ“śĪļÖ»ÓŠŅ»ÖֵĹ²ÓŠ____ÖÖ(²»ŗ¬Į¢ĢåŅģ¹¹)£¬ŗĖ“Ź²ÕńĒāĘ×ĻŌŹ¾2×é·åµÄŹĒ_____________________”£(Š“½į¹¹¼ņŹ½)£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æČēĻĀĶ¼ĖłŹ¾£¬øō°å¢ń¹Ģ¶Ø²»¶Æ£¬»īČū¢ņæÉ×ŌÓÉŅĘ¶Æ£¬M”¢NĮ½øöČŻĘ÷ÖŠ¾ł·¢Éś·“Ó¦£ŗA(g)£«3B(g)![]() 2C(g) ¦¤H£½£192 kJ”¤mol£1”£ĻņM”¢NÖŠ£¬·Ö±šĶØČėx mol AŗĶy mol BµÄ»ģŗĻĘųĢ壬³õŹ¼M”¢NČŻ»żĻąĶ¬£¬±£³ÖĪĀ¶Č²»±ä”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
2C(g) ¦¤H£½£192 kJ”¤mol£1”£ĻņM”¢NÖŠ£¬·Ö±šĶØČėx mol AŗĶy mol BµÄ»ģŗĻĘųĢ壬³õŹ¼M”¢NČŻ»żĻąĶ¬£¬±£³ÖĪĀ¶Č²»±ä”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ£Ø £©
![]()
A. ČōĘ½ŗāŹ±AĘųĢåŌŚĮ½ČŻĘ÷ÖŠµÄĢå»ż·ÖŹżĻąµČ£¬ŌņxŅ»¶ØµČÓŚy
B. Čōx”Ćy£½1”Ć2£¬ŌņĘ½ŗāŹ±£¬MÖŠµÄ×Ŗ»ÆĀŹ£ŗA>B
C. Čōx”Ćy£½1”Ć3£¬µ±MÖŠ·Å³öČČĮæ172.8 kJŹ±£¬AµÄ×Ŗ»ÆĀŹĪŖ90%
D. Čōx£½1.2£¬y£½1£¬NÖŠ“ļµ½Ę½ŗāŹ±Ģå»żĪŖ2 L£¬ŗ¬ÓŠC 0.4 mol£¬ŌŁĶØČė0.36 mol AŹ±£¬vÕż<vÄę
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA”¢B”¢C”¢D”¢E “ś±ķĪåÖÖŌŖĖŲ”£ĒėĢīæÕ£ŗ
(1)A ŌŖĖŲ»łĢ¬Ō×ÓµÄ×īĶā²ćÓŠ 3 øöĪ“³É¶Ōµē×Ó£¬“ĪĶā²ćÓŠ 2 øöµē×Ó£¬ĘäŌŖĖŲ·ūŗÅĪŖ_____£¬ µē×ÓÅŲ¼Ķ¼ĪŖ_____£¬Ō×ÓŗĖĶāµē×ÓµÄŌĖ¶ÆדĢ¬ÓŠ_____ÖÖ”£
(2)B ŌŖĖŲµÄ-2 ¼ŪĄė×ÓŗĶ C ŌŖĖŲµÄ+1 ¼ŪĄė×ӵĵē×Ó²ć½į¹¹¶¼Óėė²Ō×ÓµÄĻąĶ¬£¬B µÄŌ×Ó½į¹¹ Ź¾ŅāĶ¼ĪŖ_____£¬B”¢C ĖłŠĪ³ÉµÄ»ÆŗĻĪļµÄµē×ÓŹ½ĪŖ_____”£
(3)D ŌŖĖŲµÄ+3 ¼ŪĄė× 3d Äܼ¶ĪŖ°ė³äĀś×“Ģ¬£¬D µÄŌŖĖŲĆū³ĘĪŖ________£¬Ę仳Ģ¬Ō×ÓµÄĖłÓŠ µē×ÓÕ¼ÓŠ________øöŌ×Ó¹ģµĄ”£
(4)E ŌŖĖŲ»łĢ¬Ō× M ²ćĪŖČ«³äĀś×“Ģ¬£¬N ²ćƻӊ³É¶Ōµē×Ó£¬Ö»ÓŠŅ»øöĪ“³É¶Ōµē×Ó£¬Ōņ E µÄ»łĢ¬Ō×ӵĵē×ÓÓŠ ________________øö Éģ Õ¹ ·½ Ļņ £¬Ę仳Ģ¬Ō×ӵĵē×ÓÅŲ¼Ź½ ĪŖ_________________________ ,E +µÄĶāĪ§µē×ÓÅŲ¼Ķ¼ĪŖ_____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĶ¼ŹĒĶعżČČ»Æѧѻ·ŌŚ½ĻµĶĪĀ¶ČĻĀÓÉĖ®»ņĮņ»ÆĒā·Ö½āÖʱøĒāĘųµÄ·“Ó¦ĻµĶ³ŌĄķ£¬ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ

A. ·“Ó¦¢ŚĪŖ·“Ó¦¢ŪĢį¹©ĮĖŌĮĻ
B. ·“Ó¦¢ŚŅ²ŹĒSO2׏Ō“ĄūÓƵķ½·ØÖ®Ņ»
C. ÖʵƵČĮæH2ĖłŠčÄÜĮæ½ĻÉŁµÄŹĒĻµĶ³(I)
D. ĻµĶ³(I)ÖĘĒāµÄČČ»Æѧ·½³ĢŹ½ĪŖH2O(l) ![]() H2(g) + 1/2O2(g) ¦¤H = +286 kJ/mol
H2(g) + 1/2O2(g) ¦¤H = +286 kJ/mol
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com