
”¾ĢāÄæ”æŅŌŹÆÓĶŗĶµķ·Ū»ņĻĖĪ¬ĖŲĪŖŌĮĻ¾łæÉÖʵĆŅŅĖįŅŅõ„£¬×Ŗ»Æ¹ŲĻµČēĻĀĶ¼£ŗ
ĘäÖŠĘųĢåBŌŚ±źæöĻĀµÄĆܶČĪŖ£ŗ1.25 g/L”£Ēė»Ų“š£ŗ
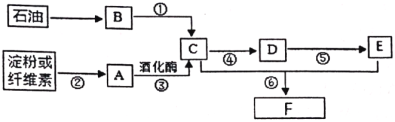
£Ø1£©BÖŠ¹ŁÄÜĶŵÄĆū³ĘŹĒ________”£
£Ø2£©AµÄ·Ö×ÓŹ½ŹĒ________”£
£Ø3£©·“Ó¦¢ÜµÄ»Æѧ·½³ĢŹ½ĪŖ________”£
£Ø4£©ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ________”£
A£®·“Ó¦¢Ś¢Ż¢ŽŹōÓŚČ”“ś·“Ó¦
B£®ĪļÖŹFµÄ½į¹¹¼ņŹ½ĪŖCH3CH2OOCCH3
C£®BŗĶEæÉŌŚŅ»¶ØĢõ¼žĻĀÖ±½ÓŗĻ³ÉF£¬øĆ·½·Ø·ūŗĻĀĢÉ«»ÆѧĄķÄī
D£®æÉÓĆŠĀÖĘĒāŃõ»ÆĶ¼ų±šC”¢D”¢EČżÖÖĪļÖŹ
”¾“š°ø”æĢ¼Ģ¼Ė«¼ü C6H12O6 2CH3CH2OH + O2![]() 2CH3CHO +2H2O BCD
2CH3CHO +2H2O BCD
”¾½āĪö”æ
ĘųĢåBŌŚ±źæöĻĀµÄĆܶČĪŖ£ŗ1.25 g/L£¬ĘäĦ¶ūÖŹĮæ = 1.25 g/L”Į22.4 L/mol = 28 g/mol£¬ĘäÖŠĘųĢåBÓÖŹĒÓÉŹÆÓĶÖĘµĆ£¬ŌņæÉĶĘ³öĘųĢåBĪŖŅŅĻ©£¬øł¾Ż·“Ó¦¢ŚæÉÖŖĪļÖŹAĪŖĘĻĢŃĢĒ£¬¾¹ż¾Ę»ÆĆø×÷ÓĆ»įÉś³ÉŅŅ“¼£¬ŌņCĪŖŅŅ“¼£¬ŅŅ“¼¾¹ż·“Ó¦¢Ü±»Ńõ»Æ³ÉŅŅČ©£¬ŌņDĪŖŅŅČ©£¬¼ĢŠų±»Ńõ»Æ·¢Éś·“Ó¦¢Ż£¬Éś³ÉŅŅĖį£¬ŌņEĪŖŅŅĖį£¬ŅŅČ©ÓėŅŅĖįŌŚŅ»¶ØĢõ¼žĻĀ·¢Éśõ„»Æ·“Ӧɜ³ÉŅŅĖįŅŅõ„£¬ŌņFĪŖŅŅĖįŅŅõ„£¬¾Ż“Ė·ÖĪö×÷“š”£
øł¾ŻÉĻŹö·ÖĪö£¬Ōņ
£Ø1£©BĪŖŅŅĻ©£¬Ęä·Ö×ÓÄŚ¹ŁÄÜĶÅĆū³ĘĪŖĢ¼Ģ¼Ė«¼ü£¬
¹Ź“š°øĪŖĢ¼Ģ¼Ė«¼ü£»
£Ø2£©AĪŖĘĻĢŃĢĒ£¬Ęä·Ö×ÓŹ½ĪŖC6H12O6£¬
¹Ź“š°øĪŖ£ŗC6H12O6£»
£Ø3£©·“Ó¦¢ÜĪŖŅŅ“¼ŌŚĶ»ņŅų¼ÓČȵÄĢõ¼žĻĀ±»Ńõ»ÆŅŅČ©µÄ¹ż³Ģ£¬Ęä¶ŌÓ¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ2CH3CH2OH + O2![]() 2CH3CHO +2H2O£¬
2CH3CHO +2H2O£¬
¹Ź“š°øĪŖ£ŗ2CH3CH2OH + O2![]() 2CH3CHO +2H2O£»
2CH3CHO +2H2O£»
£Ø4£©A. ·“Ó¦¢ŚĪŖµķ·ŪµÄĖ®½ā·“Ó¦£¬ŹōÓŚČ”“ś·“Ó¦£»·“Ó¦¢ŻĪŖŅŅČ©±»Ńõ»ÆĪŖŅŅĖįµÄ¹ż³Ģ£¬ŹōÓŚŃõ»Æ·“Ó¦£¬·“Ó¦¢ŽĪŖŅŅĖįŅŅõ„µÄõ„»Æ·“Ó¦£¬ŹōÓŚČ”“ś·“Ó¦£¬¹ŹAĻī“ķĪó£»
B. ĪļÖŹFŹĒĪŖŅŅĖįŅŅõ„£¬ĪŖŅŅĖįÓėŅŅ“¼ĶعżĖįĶŃōĒ»ł“¼ĶŃĒāµĆµ½µÄÖ÷ŅŖ²śĪļ£¬Ęä·Ö×ӵĽį¹¹¼ņŹ½ĪŖCH3CH2OOCCH3£¬¹ŹBĻīÕżČ·£»
C. BĪŖŅŅĻ©£¬EĪŖŅŅĖį£¬Į½ÕßÖ±½Ó»ÆŗĻ£¬ĪŽø±²śĪļ£¬Ō×ÓĄūÓĆĀŹĪŖ100%£¬·ūŗĻĀĢÉ«»ÆѧĄķÄī£¬¹ŹCĻīÕżČ·£»
D. ŠĀÖĘĒāŃõ»ÆĶÓėŅŅ“¼»„ČÜ£¬ÓėŅŅČ©»į·¢Éś·“Ӧɜ³ÉשŗģÉ«³Įµķ£¬ÓėŅŅĖį·¢ÉśĖį¼īÖŠŗĶ£¬Šü×ĒŅŗ±äĄ¶É«³ĪĒåČÜŅŗ£¬ŌņæÉÓĆŠĀÖĘĒāŃõ»ÆĶ¼ų±šC”¢D”¢EČżÖÖĪļÖŹ£¬¹ŹDĻīÕżČ·£»
“š°øŃ”B”¢C”¢D”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ£Ø1£©ĻĀĮŠø÷×éĪļÖŹ£ŗ
A ½šøÕŹÆÓėŹÆÄ«£»B µķ·ŪÓėĻĖĪ¬ĖŲ£»C ėÓėė®£»D ¼×ĶéÓėĪģĶ飻 E ĘĻĢŃĢĒÓė¹ūĢĒ
F![]() Óė
Óė G
G  Óė
Óė
ĘäÖŠ»„ĪŖĶ¬Ī»ĖŲ _____£»£ØĢī±ąŗÅ£®ĻĀĶ¬£©£¬»„ĪŖĶ¬ĻµĪļµÄŹĒ____£¬»„ĪŖĶ¬·ÖŅģ¹¹ĢåµÄŹĒ______£¬ŹĒĶ¬Ņ»ÖÖĪļÖŹµÄŹĒ________£®
£Ø2£©»ÆŗĻĪļAµÄ½į¹¹¼ņŹ½ĪŖ£ŗ £¬ĖüŹĒĘūÓĶČ¼ÉÕĘ·ÖŹæ¹ÕšŠŌÄܵIJĪÕÕĪļ£¬ĘäÖŠAµÄĶ¬·ÖŅģ¹¹ĢåÖŠŗ¬µČŠ§ĒāŌ×ÓÖÖĄą×īÉŁµÄŅ»ÖÖ½į¹¹¼ņŹ½ĪŖ£ŗ_____£»ČōAŹĒÓÉĻ©ĢžŗĶH2Ķعż¼Ó³É·“Ó¦µĆµ½£¬ŌņøĆĻ©ĢžµÄĖłÓŠæÉÄܵĽį¹¹¼ņŹ½ĪŖ_____”£
£¬ĖüŹĒĘūÓĶČ¼ÉÕĘ·ÖŹæ¹ÕšŠŌÄܵIJĪÕÕĪļ£¬ĘäÖŠAµÄĶ¬·ÖŅģ¹¹ĢåÖŠŗ¬µČŠ§ĒāŌ×ÓÖÖĄą×īÉŁµÄŅ»ÖÖ½į¹¹¼ņŹ½ĪŖ£ŗ_____£»ČōAŹĒÓÉĻ©ĢžŗĶH2Ķعż¼Ó³É·“Ó¦µĆµ½£¬ŌņøĆĻ©ĢžµÄĖłÓŠæÉÄܵĽį¹¹¼ņŹ½ĪŖ_____”£
£Ø3£©ĄŗĶé·Ö×ӵļüĻߏ½ČēĶ¼ĖłŹ¾£¬ŹŌ»Ų“š£ŗ

Š“³öĄŗĶé·Ö×ӵĻÆѧŹ½________£»ĄŗĶé·Ö×ÓµÄŅ»ĀČČ”“śĪļµÄÖÖŹżĪŖ______ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æA”¢B”¢C”¢D¾łĪŖ¶ĢÖÜĘŚŌŖĖŲ£¬ĖüĆĒŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼ĖłŹ¾£¬ĘäÖŠBµÄµ„ÖŹŌŚæÕĘųÖŠŗ¬ĮæŌ¼Õ¼80£„”£
A | B | C | |||
D |
£Ø1£©Š“³öĻĀĮŠŌŖĖŲµÄĆū³Ę£ŗC____£¬D___”£
£Ø2£©»³öBµÄŌ×Ó½į¹¹Ź¾ŅāĶ¼____”£CŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĪ»ÖĆŹĒ____”£
£Ø3£©B”¢CĮ½ÖÖŌŖĖŲ×ī¼ņµ„Ēā»ÆĪļµÄĪČ¶ØŠŌÓÉĒæµ½ČõµÄĖ³ŠņŹĒ_____”£Š“³öAµÄ×ī¼ņµ„Ēā»ÆĪļµÄµē×ÓŹ½£ŗ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æµĀ¹ś»Æѧ¼ŅææāĄÕČĻĪŖ£ŗ±½·Ö×ÓŹĒÓÉ6øöĢ¼Ō×ÓŅŌµ„Ė«¼üĻą»„½»Ģę½įŗĻ¶ų³ÉµÄ»·×“½į¹¹£¬ĪŖĮĖŃéÖ¤ææāĄÕÓŠ¹Ų±½»·µÄ¹Ūµć£¬¼×Ķ¬Ń§Éč¼ĘĮĖČēĶ¼ŹµŃé·½°ø”£

¢Ł°“ČēĶ¼ĖłŹ¾µÄ×°ÖĆĶ¼Į¬½ÓŗĆø÷ŅĒĘ÷£»
¢Ś¼ģŃé×°ÖƵÄĘųĆÜŠŌ£»
¢ŪŌŚAÖŠ¼ÓČėŹŹĮæµÄ±½ŗĶŅŗäåµÄ»ģŗĻŅŗĢ壬ŌŁ¼ÓČėÉŁĮæĢś·Ū£¬ČūÉĻĻšĘ¤Čū£¬“ņæŖÖ¹Ė®¼ŠK1”¢K2”¢K3£»
¢Ü“żCÖŠÉÕĘæŹÕ¼ÆĀśĘųĢåŗ󣬽«µ¼¹ÜbµÄĻĀ¶Ė²åČėÉÕ±ĄļµÄĖ®ÖŠ£¬¼·Ń¹Ō¤ĻČ×°ÓŠĖ®µÄ½ŗĶ·µĪ¹ÜµÄ½ŗĶ·£¬¹Ū²ģŹµŃéĻÖĻó”£
Ēė»Ų“šĻĀĮŠĪŹĢā”£
(1)AÖŠĖł·¢Éś·“Ó¦µÄ·“Ó¦·½³ĢŹ½ĪŖ_____£¬ÄÜÖ¤Ć÷ææāĄÕ¹Ūµć“ķĪóµÄŹµŃéĻÖĻóŹĒ_____”£
(2)×°ÖĆBµÄ×÷ÓĆŹĒ_________”£
(3)CÖŠÉÕĘæµÄČŻĘ÷ĪŖ500 mL£¬ŹÕ¼ÆĘųĢåŹ±£¬ÓÉÓŚæÕĘųĪ“Åž”£¬×īÖÕĖ®Ī“³äĀśÉÕĘ棬¼ŁÉčÉÕĘæÖŠ»ģŗĻĘųĢå¶ŌH2µÄĻą¶ŌĆܶČĪŖ37.9£¬ÄĒĆ“ŹµŃé½įŹųŹ±£¬æɼĘĖć½ųČėÉÕĘæÖŠµÄĖ®µÄĢå»żĪŖ_______mL”£(æÕĘųµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæĪŖ29)
(4)ŅŃÖŖČéĖįµÄ½į¹¹¼ņŹ½ĪŖ£ŗ![]() ”£ŹŌ»Ų“š£ŗ ČéĖįøśĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ______”£
”£ŹŌ»Ų“š£ŗ ČéĖįøśĒāŃõ»ÆÄĘČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æijĪŽÉ«ČÜŅŗÖŠŗ¬ÓŠK+”¢Cl£”¢OH£”¢SO![]() ”¢SO
”¢SO![]() £¬ĪŖ¼ģŃéČÜŅŗÖŠĖłŗ¬µÄijŠ©ŅõĄė×Ó£¬ĻŽÓƵďŌ¼ĮÓŠ£ŗŃĪĖį”¢ĻõĖį”¢ĻõĖįŅųČÜŅŗ”¢ĻõĖį±µČÜŅŗ”¢äåĖ®ŗĶ·ÓĢŖČÜŅŗ”£¼ģŃéĘäÖŠOH£µÄŹµŃé·½·ØŹ”ĀŌ£¬¼ģŃéĘäĖūŅõĄė×ӵĹż³ĢČēĻĀĶ¼ĖłŹ¾”£
£¬ĪŖ¼ģŃéČÜŅŗÖŠĖłŗ¬µÄijŠ©ŅõĄė×Ó£¬ĻŽÓƵďŌ¼ĮÓŠ£ŗŃĪĖį”¢ĻõĖį”¢ĻõĖįŅųČÜŅŗ”¢ĻõĖį±µČÜŅŗ”¢äåĖ®ŗĶ·ÓĢŖČÜŅŗ”£¼ģŃéĘäÖŠOH£µÄŹµŃé·½·ØŹ”ĀŌ£¬¼ģŃéĘäĖūŅõĄė×ӵĹż³ĢČēĻĀĶ¼ĖłŹ¾”£

£Ø1£©Ķ¼ÖŠŹŌ¼Į¢Ł”«¢ŻČÜÖŹµÄ»ÆѧŹ½·Ö±šŹĒ
¢Ł________£¬¢Ś________£¬¢Ū________£¬¢Ü__________£¬¢Ż__________”£
£Ø2£©Ķ¼ÖŠĻÖĻóa”¢b”¢c±ķĆ÷¼ģŃé³öµÄĄė×Ó·Ö±šŹĒa________”¢b________”¢c________”£
£Ø3£©°×É«³ĮµķA¼ÓŹŌ¼Į¢Ś·“Ó¦µÄĄė×Ó·½³ĢŹ½_________________”£
£Ø4£©ĪŽÉ«ČÜŅŗC¼ÓŹŌ¼Į¢ŪµÄÖ÷ŅŖÄæµÄŹĒ_____________________”£
£Ø5£©°×É«³ĮµķAČō¼ÓŹŌ¼Į¢Ū¶ų²»¼ÓŹŌ¼Į¢Ś£¬¶ŌŹµŃéµÄÓ°ĻģŹĒ____________________”£
£Ø6£©ĘųĢåEĶØČėŹŌ¼Į¢Ü·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½ŹĒ____________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŌŚ±ź×¼×“æöĻĀCH4”¢H2S”¢NH3¾łĪŖĘųĢ壬·Ö±šÓŠ¢Ł11.2L H2S¢Ś16g CH4¢Ū1.204”Į1024øöNH3·Ö×Ó£¬ĻĀĮŠĪļĄķĮæ“óŠ”±Č½ĻÕżČ·µÄŹĒ£Ø £©
A. Ģå»ż£ŗ¢Ś£¾¢Ū£¾¢Ł
B. ĆÜ¶Č£ŗ¢Ū£¾¢Ś£¾¢Ł
C. ÖŹĮæ£ŗ¢Ū£¾¢Ś£¾¢Ł
D. Ō×Ó×ÜŹż£ŗ¢Ū£¾¢Ś£¾¢Ł
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”抔ĶõĶ¬Ń§×¼±øÓĆCuSO4”¤5H2OÅäÖĘ500mL 1 mol/LµÄČÜŅŗ”£
£Ø1£©Š”ĶõĶ¬Ń§Ń”ÓĆµÄ²£Į§ŅĒĘ÷ÓŠ£ŗÉÕ±£¬²£Į§°ō£¬__________£»________”£
£Ø2£©²£Į§°ōŌŚøĆŹµŃéÖŠÓŠÖŲŅŖµÄÓĆĶ¾£¬·Ö±šŹĒ__________ŗĶ _________£»
£Ø3£©Š”ĶõĶ¬Ń§Ķعż¼ĘĖć£¬ÓĆĶŠÅĢĢģĘ½³ĘČ”________gCuSO4”¤5H2O”£
£Ø4£©ĪļÖŹµÄĮæÅضČĪó²ī£ØĢīĘ«øß”¢Ę«µĶ”¢ĪŽÓ°Ļģ£©
¢ŁČōČŻĮæĘæÖŠĻ“¾»ŗóĪ“øÉŌļ£¬²ŠĮōÉŁĮæĖ®£¬ŌņĖłÅäÖʵÄČÜŅŗÅØ¶Č½«_________£»
¢Ś¶ØČŻŹ±£¬ČōŃŪ¾¦ø©ŹÓ£¬ŌņĖłÅäÖʵÄČÜŅŗÅØ¶Č½«___________£»
¢ŪŅ”ŌČŗóŅŌĆāµĶÓŚæĢ¶ČĻߣ¬ŌŁ¼ÓČėÉŁĮæĖ®________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĮŖ°±(ÓÖ³ĘėĀ£¬N2H4£¬ĪŽÉ«ŅŗĢå)ŹĒŅ»ÖÖÓ¦ÓĆ¹ć·ŗµÄ»Æ¹¤ŌĮĻ£¬æÉÓĆ×÷»š¼żČ¼ĮĻ”£
£Ø1£©ĮŖ°±·Ö×ӵĵē×ÓŹ½ĪŖ______________£¬
£Ø2£©ĮŖ°±ĪŖ¶žŌŖČõ¼ī£¬ŌŚĖ®ÖŠµÄµēĄė·½Ź½Óė°±ĻąĖĘ”£Š“³öŌŚĖ®ÖŠĮŖ°±µŚŅ»²½µēĄė·“Ó¦µÄ·½³ĢŹ½______________________________________________________”£
£Ø3£©ŅŃÖŖ12.8 gµÄŅŗĢ¬øßÄÜČ¼ĮĻĮŖ°±ŌŚŃõĘųÖŠČ¼ÉÕ£¬Éś³ÉĘųĢ¬N2ŗĶŅŗĢ¬Ė®£¬·Å³ö248.8kJµÄČČĮ攣Š“³ö±ķŹ¾ŅŗĢ¬ĮŖ°±Č¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½___________________”£
£Ø4£©ŅŃÖŖ¢Ł2O2(g)+N2(g)=N2O4(l) £»¦¤H1
¢ŚN2(g)+2H2(g)=N2H4(l) £»¦¤H2
¢ŪO2(g)+2H2(g)=2H2O(g) £» ¦¤H3
¢Ü2 N2H4(l) + N2O4(l)= 3N2(g)+ 4H2O(g) £»¦¤H4
ÉĻŹö·“Ó¦ČČŠ§Ó¦Ö®¼äµÄ¹ŲĻµŹ½ĪŖ¦¤H4=_________________£¬ĮŖ°±ŗĶN2O4æÉ×÷ĪŖ»š¼żĶĘ½ų¼ĮµÄÖ÷ŅŖŌŅņĪŖ____________________________________________ £ØÖĮÉŁ“š2µć£©”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĻĀĮŠÓŠ¹ŲĆŗµÄĖµ·ØÕżČ·µÄŹĒ( )
A.ĆŗµÄøÉĮó”¢Ęų»ÆŗĶŅŗ»Æ¶¼ŹĒĪļĄķ±ä»Æ
B.ĆŗÖŠŗ¬ÓŠ±½”¢¼×±½”¢¶ž¼×±½µČ·¼ĻćĢž
C.ĶعżĆŗµÄøÉĮóæÉ»ńµĆ±½”¢¼×±½µČ·¼ĻćĢž
D.Ė®ĆŗĘųŹĒĶعżĆŗµÄøÉĮóµĆµ½µÄĘųĢåČ¼ĮĻ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com