
(1)��A��B��һ��������ϣ�1 L���������ȫȼ�պ���ͬ��ͬѹ�µõ�3.6 LCO2,���ƶϸû������Ŀ�����ɼ�A��B���ʱ������ȣ������������С�
A�Ļ�ѧʽ | B�Ļ�ѧʽ | VA��VB |
|
|
|
|
|
|
|
|
|
|
|
|
(2)1 mol�û������ǡ����ʹ��0.4 mol������Ȼ�̼��Һ��ȫ��ɫ���ƶ�����������з��ϸ�������A��B�Ļ�ѧʽ��
(3)120 ��ʱ��1 L A��1 L B������O2��Ϻ��ȼ����ͬ��ͬѹ���������2 L����ͨ������ȷ��A��B�Ļ�ѧʽ��
����������1 L�������ȼ�յ�CO2����3.6 L,˵����������ƽ������ʽ��̼ԭ��Ϊ3.6 ����
(1)Aֻ����ΪC4H10 B����ΪC2H4 C2H2 C3H6 C3H4

(2)�����Ȳ������ˮ�ӳɣ�ȲӦΪ0.2 mol�����n(Ȳ)��n(Br2)=1��2�������ϩ������ˮ�ӳɣ�ϩӦΪ0.4 mol����n(ϩ)��n(Br2)=1��1��
![]()
1 ![]() x
x ![]() y
y ![]()
2 L 2 L
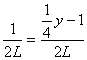 ��y=8��ƽ������ʽ����ԭ��Ϊ8����
��y=8��ƽ������ʽ����ԭ��Ϊ8����
�𰸣�(1)���±�
C4H10 | C2H4 | 4��1 |
C4H10 | C2H2 | 4��1 |
C4H10 | C3H6 | 3��2 |
C4H10 | C3H4 | 3��2 |
(2)A��C4H10 B��C2H2��A��C4H10 B��C3H6
(3)A��C4H10 B��C3H6



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������й��ƶ�ϩ���Ʋⲻ��ȷ���ǣ�������
�������й��ƶ�ϩ���Ʋⲻ��ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʯ���ѽ���ҪĿ���ǵõ������IJ������� | B����C18�������������;������ѻ����Եõ����� | C��ú��������Һ�����ǻ�ѧ�仯 | D��ú�к��б��ͼױ��������ȸ�������ķ��������Ƿ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������ϩ������ϩ���Dz������� | ||
B���۱���ϩ�Ľṹ��ʽΪ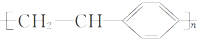 | ||
C������ϩ��ȡ������ϩ�ķ�ӦΪnCH2�TCHCl
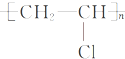 | ||
| D����ϩ�;���ϩ������������Ȼ�̼��Һ�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��ˮ��һ��2009��2010ѧ��߶���ѧ����ĩ���Ի�ѧ�Ծ�(��) ���ͣ�038
����A�Ͳ�������B��Ϊ���壬��A���е�̼ԭ��������B��
(1)��A��B��һ��������ϵ�1 L������壬��ȫȼ�պ�õ�ͬ�����µ�3.6 LCO2���ƶϻ������Ŀ�����ɼ�����ȣ�������������±���

(2)120��ʱ��1 L��A��1 L��B������������ϵ�ȼ����ͬ��ͬѹ�����������2 L����ͨ������ȷ��A��B�Ļ�ѧʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com