
���� �ٸ���n=cV��m=nM��������Ҫ���������Ƶ��������������õ������Dz�������
������ƿֻ��һ���̶��ߣ�ֻ�������������Ӧ�����������Һ���������Ƶ���500mL��0.1mol/L��NaOH��Һ����ѡ����ʵ�����ƿ��
�۶���ʱ�õ������ǽ�ͷ�ιܣ�
��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳���������
��2������C=$\frac{1000�Ѧ�}{M}$�����㣻
��3��������Һϡ�Ͷ���CŨVŨ=CϡVϡ�����㣻
��4������c=n/V��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺����Ҫ���������Ƶ����ʵ���n=cV=0.1mol/L��0.5L=0.05mol������m=nM=0.05mol��40g/mol=2.0g���������õ������Dz�������
�ʴ�Ϊ��2.0����������
������ƿֻ��һ���̶��ߣ�ֻ�������������Ӧ�����������Һ���������Ƶ���500mL0.1mol/L��NaOH��Һ����ѡ�������ƿΪ500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
�۶���ʱ��������ƿ��עˮ��Һ�����̶���1��2cm�������ý�ͷ�ι�С�ĵμ�����ˮ����Һ��Һ����͵���̶������У�
�ʴ�Ϊ����ͷ�ιܣ�
��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ���˳���Ǣ٢ڢܢۢݣ�
�ʴ�Ϊ���٢ڢܢۢݣ�
��2������Һ����������Ϊ�أ�����������Һ�����ʵ���Ũ��C=$\frac{1000�Ѧ�}{M}$=$\frac{1000mL/L��1.06g/mL����}{40g/mol}$=0.1mol/L����æ�=0.0038��0.38%��
�ʴ�Ϊ��0.38%��
��3��������������Һ�У�c��NaOH��=c��Na+��=0.01mol/L����ϡ�ͺ���Һ�����ΪVmL��������Һϡ�Ͷ���CŨVŨ=CϡVϡ��֪��
20mL��0.1mol/L=0.01mol/L��VmL�����V=200mL���ʴ�Ϊ��200��
��4�����������������׳��⣬����ֽ����NaOH�ᵼ���������Ƴ����Һ��ѽ���ֽ�ϵ���������ȫ�������ձ��������������Ƶ���ʧ������Ũ��ƫ�ͣ��ʢ�ѡ��
�ڽ��ձ��е���Һת�Ƶ�����ƿʱ������������ƿ�⣬�ᵼ�����ʵ���ʧ��Ũ��ƫ�ͣ��ʢ�ѡ��
�۶���ʱ���ӿ̶��ߣ�������Һ�����ƫС��Ũ��ƫ�ߣ��ʢ۲�ѡ��
�ܶ���ʱ���ӿ̶��ߣ�������Һ���ƫ��Ũ��ƫ�ͣ��ʢ�ѡ��
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʢݲ�ѡ��
��ѡ�٢ڢܣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
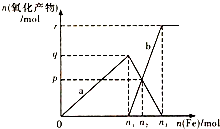 ����4molHNO3��ϡ����ֱ��벻ͬ���������۷�Ӧ��������������a��b���������ʵ�����ϵ��ͼ��ʾ�������й��жϲ���ȷ���ǣ�������
����4molHNO3��ϡ����ֱ��벻ͬ���������۷�Ӧ��������������a��b���������ʵ�����ϵ��ͼ��ʾ�������й��жϲ���ȷ���ǣ�������| A�� | a��Fe��NO3��3 | B�� | n1=1 | C�� | p=1.2 | D�� | n3=1.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �¶�/�� | ��ʼ����ʵ���/mol | ƽ�����ʵ���/mol | |||
| �������� | CO | Cl2 | COCl2 | COCl2 | |
| �� | 500 | 1.0 | 1.0 | 0 | 0.8 |
| �� | 500 | 1.0 | a | 0 | 0.5 |
| �� | 600 | 0.5 | 0.5 | 0.5 | 07 |
| A�� | ����I��ǰ5min��ƽ����Ӧ����v��CO��=0.16mol•L-1•min-1 | |
| B�� | �÷�Ӧ����ӦΪ���ȷ�Ӧ | |
| C�� | ����������ʼʱCl2�����ʵ���Ϊ0.55mol | |
| D�� | ����ʼʱ������I����CO0.8mol��Cl20.8mol���ﵽƽ��ʱCOת���ʴ���80%�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
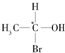 �ȣ������л������д��ڶ�ӳ�칹����ǣ�������
�ȣ������л������д��ڶ�ӳ�칹����ǣ�������| A�� | CH3COOH | B�� | CH3COCH3 | ||
| C�� | 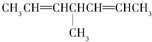 | D�� | 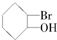 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | K+��Na+��Cl-��HCO3-��OH- | B�� | Cl-��NO3-��MnO4-��K+��Na+ | ||
| C�� | SO42-��K+��Mg2+��Cl-��NO3- | D�� | H+��Cl-��Br-��HS-��K+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ֻ�Т� | B�� | �ٺ͢� | C�� | �٢ڢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
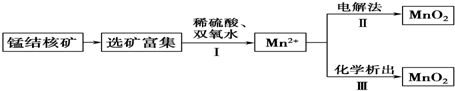
| A�� | ��Ӧ������ӷ���ʽΪMnO2+H2O2+2H+�TMn2++2H2O+O2�� | |
| B�� | ��Ӧ����������ӦʽΪMn2+-2e-+2H2O�TMnO2��+4H+ | |
| C�� | ������KClO3����Ӧ��Ϊ2ClO3-+5Mn2++4H2O�T5MnO2��+Cl2��+8H+ | |
| D�� | ������KMnO4����Ӧ��Ϊ3Mn2++2MnO4-+2H2O�T5MnO2��+4H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
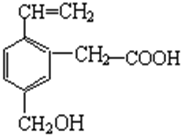
| A�� | �ܸ�NaOH��Һ��Ӧ | B�� | ��ʹ����KMnO4��Һ��ɫ | ||
| C�� | �ʼ��� | D�� | �ܷ���������Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CCl4������CH4�Ƶã�����������ȡ��ˮ�еĵ� | |
| B�� | ��ȵ����ʵ�������ϩ�ͼ�����ȫȼ�պ������ˮ��������ͬ | |
| C�� | ����ϩ��HCl��Ӧ���Ʊ����������������������Ӧ���� | |
| D�� | ����Ŀռ乹�����������壬���Զ��ȼ��������ֲ�ͬ�Ľṹ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com