
����Ҫ�ɷ��Ǵ��ᣬ�����ճ��������кܶ����á�
(1)����ʱ��������ʳ���Ͼƿ���ʹ���Ƶ�������������ζ��������ζ������________��
A��ʳ��
B��ʳ���е�����
C���Ͼ��е��Ҵ�
D���Ͼ��е��Ҵ���ʳ���е����ᷴӦ���ɵ���������
(2)��ˮ���þ��˻����ڱڲ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ���ڼ���ʱ����е���״ף����ɳ�ȥˮ����д����һ�����з�����Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
(1)A�����ڱ��е�λ����________��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ��
__________________________��
(2)B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ__________����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________________���ڲ����м�������KI����Ӧ�����CCl4�����л�����______ɫ��
(3)����ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ������A�Ķ�Ԫ���ӻ����� |
| b | �����зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1��1 |
| c | ����ѧ���ΪBDF2 |
| d | ��ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
a�Ļ�ѧʽΪ________��b�Ļ�ѧʽΪ______________��c�ĵ���ʽΪ________��d�ľ���������________��
(4)��A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ��________������ ���п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ__________��
���п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ת���У�A��һ�����Σ�D����Է���������C����Է���������16��E���ᣬYΪ���ʣ���X������ǿ�ỹ��ǿ��ʱ���������µ�ת����ϵ��
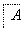
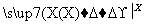
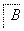
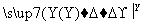
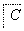
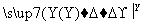
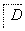
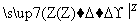

��X��ǿ��ʱ��A��B��C��D��E������ͬһ��Ԫ�أ���X��ǿ��ʱ��A��B��C��D��E����������ͬһ��Ԫ�ء���ش�
(1)A��________��Y��________��Z��________��
(2)��X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��_______________ _________________________________________________________��
(3)��X��ǿ��ʱ��E��________��д��B����C�Ļ�ѧ����ʽ��_______________ _________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ1����ҺA�п��ܺ���Mg2����Cu2����Al3����Fe2�����������ӣ�BΪ����ɫ���壬����E��������ų��Ͱ�ɫ�������ɣ�����ɫ��������ʵ��������������������ϵ��ͼ2��
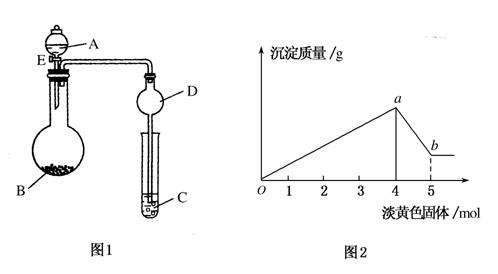
(1)����Һ�к��е���������________��
(2)д��ͼ2����a��b�η�Ӧ�����ӷ���ʽ________________��________________��
(3)A��Һ�д��ڵ������ӵ����ʵ���Ũ��֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й�������������У�����ȷ����(����)
A���������ʽ��C2H4O2����4����ԭ�ӣ�����һԪ��
B�������Ǿ���ǿ�Ҵ̼�����ζ��Һ��
C������������ˮ���Ҵ�
D������������Ҵ����Լ�������̼��������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ֲ���ͳ�Ϊ����ȡ���зḻ����֬�����з�����ƺ�������(����)
A��������ˮ���ݣ�ʹ���е���֬����ˮ��Ȼ���ٷ���
B���Ƚ���ѹ�ɿ���״�����������л��ܼ����ݣ�Ȼ��Խ���Һ�����������
C�������ü���Һ������ʹ���е���֬�ܽ�������Ȼ��������
D�������飬Ȼ������������ȣ�ʹ���е���֬�ӷ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����Ͷ�ʽ���һ����ҵ�ƾ�����Ŀ�����ù�ҵ�ƾ������ͻ���Ƴɡ��Ҵ����͡����Խ�ʡʯ����Դ����֪�ƾƾ��ķ��������֣�
���ڴ�����������ϩ��ˮ��Ӧ��
��CH3CH2Br��H2O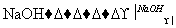 CH3CH2OH��HBr��
CH3CH2OH��HBr��
��(C6H10O5)n(����)��nH2O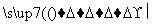 nC6H12O6(������)��
nC6H12O6(������)��
C6H12O6(������)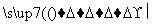 2C2H5OH��2CO2����
2C2H5OH��2CO2����
(1)�����ٵĻ�ѧ����ʽ��________________________________________��
(2)�����ڵĻ�ѧ��Ӧ������____________��
(3)Ϊ����ʯ�Ͷ�ȱ��������ԴΣ��������Ϊ����Ӧѡ����һ�ַ���������ҵ�ƾ������������
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
(4)�������ɫ��ѧ(��ԭ�������ʡ����)�ĽǶȿ����ƾƾ���õ�һ�鷽����________��
A���� B����
C���٢� D���٢ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�������Թ�ҵ��ˮ�к���һ������Fe3����Cu2����Au3�������ӡ����������ͼ�еĹ������̣����ó��õ��ᡢ���ҵ�����еķ���м���ӷ�ˮ�л��ս𣬲�����һ���������������ͭ��
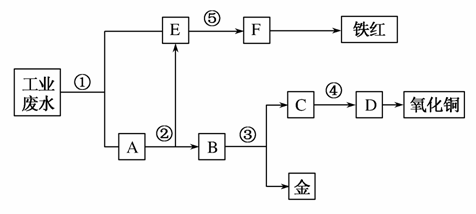
��д����հס�
(1)ͼ�б�Ŵ���������Ӧ���ʷֱ��Ǣ�________����________����________����________����________��
(2)д���ٴ�������Ӧ�����ӷ���ʽ_____________________ _____________��д���۴�������Ӧ�Ļ�ѧ����ʽ___________��
(3)����Ļ�ѧʽΪ________________________���ֱ�д�����������ͭ�ڹ�ҵ�ϵ�һ����Ҫ��;������________________������ͭ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ���ǣ�������
| �� | A�� | �ƺ���ˮ��Ӧ��Na+2H2O=Na++2OH﹣+H2�� |
| �� | B�� | AlCl3��Һ�м��������Ũ��ˮ��Al3++3NH3•H2O=Al��OH��3��+3NH4+ |
| �� | C�� | ��С�մ�����θ����ࣺCO32﹣+2H+=H2O+CO2�� |
| �� | D�� | ��FeCl3��Һ��ʴӡˢ��·�壺Fe3++Cu=Fe2++Cu2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com