
| ʵ��� ���� | ������Һ��� V/mL | ����������Һ��� V/mL |
| 1 | 19.90 | 10.00 |
| 2 | 20.10 | 10.00 |
| 3 | 22.00 | 10.00 |
| 4 | 20.00 | 10.00 |
���� ��2���ٸ��ݼ�����Һʢ���ڼ�ʽ�ζ����У�
�ڸ��ݵζ�ʱ������ע����ƿ����Һ��ɫ�ı仯����Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
��3������C�����⣩=C��������V��������V�����⣩���㣬V�����������ε�ƽ��ֵ������m=CVM����250mL�ռ���Ʒ���������Ƶ��������ٸ�������������ʽ�����������Ƶ�����������
��4��A����������ƿ���ζ��ܵĹ��켰��ȷʹ�÷������з�����
B��ʵ����û��80mL����ƿ����Ҫѡ��100mL����ƿ���Ƹ�Ũ�ȵ���Һ��
C������ƿ�к�����������ˮ��Ӱ�죻
D���ζ���û�б���Һ��ϴ���ᵼ�±�Һ��ϡ�ͣ��ζ�ʱ���ĵı�Һ���ƫ��
E��������Һʱ������ʱ���Ӷ�������Һ���ƫС��
F���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ����������ĵı���Һ���ƫ��
G����ƿ������ˮϴ��������������ˮ��������ʵ������Ӱ�죻
H���ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ������Һ�����ȡ��ֵ����
��� �⣺���������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�250mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ�������ǣ�������ƽ��ҩ�ס���������250mL����ƿ����ͷ�ιܣ�
��2���ٴ���Һ�Ǽ�����Һ��Ӧʢ���ڼ�ʽ�ζ����У�ͨ���ζ�����ȡ10.00mL����Һ��
�ʴ�Ϊ����ʽ�ζ��ܣ�
�ڵζ�ʱ������ע����ƿ����Һ��ɫ�ı仯���Ա�ȷ�ж��յ�ĵ���ζ�ʱ�����������һ�����ᣬ��Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
�ʴ�Ϊ����ƿ����Һ��ɫ�ı仯����Һ�ɺ�ɫ��Ϊ��ɫ���Ұ�����ڲ��ָ���
��3��������ʵ�����ĵ���������ƫ����ȥ����V������=$\frac{19.90+20.10+20.00}{3}$=20.00mL��
C�����⣩=$\frac{0.2010mol/L��0.0200L}{0.0100L}$=0.4020mol•L-1��
m=CVM=0.4020mol•L-1��0.25L��40g/mol=4.020g
�أ�NaOH��=$\frac{4.020g}{4.10g}$��100%=98.05%��
�ʴ�Ϊ��0.4020mol•L-1��98.05%��
��4��A������ƿ��ƿ��������ʱ��Ҫҡ�ȣ�����ʹ��ǰ�������Ƿ�©ˮ���ζ��������������ܣ�ʹ��ǰ�������Ƿ�©Һ����A��ȷ��
B������ʵ������û��60mL����ƿ��������Ҫѡ��100mL����ƿ����1mol/L����Һ����B��ȷ��
C����������Һʱ��Ҫ������ˮ���ݣ���������ƿ�к�����������ˮ��Ӱ�죬��C����
D���ζ�����ʢ���������Һʱ������ϴ������Ὣ��װҺϡ�ͣ��磺��ʽ�ζ���������ˮϴ�Ӻ�װ���Ũ�ȵ�ϡ���ᣬ���±�ҺŨ�ȼ�С���ζ�ʱ���ĵı�Һ���ƫ��õ�NaOH��Һ��Ũ�Ƚ�ƫ��D��ȷ��
E��������Һʱ������ʱ���Ӷ�������Һ���ƫС������c=$\frac{n}{V}$��֪Ũ��ƫС����E����
F���к͵ζ�ʱ���������һ�ζ���ʱ���Ӷ����������ĵı���Һ���ƫС�����õ�NaOH��Һ��Ũ�Ƚ�ƫС����F����
G����ƿ������ˮϴ��������������ˮ������������Ҫ����ˮ���̶ȣ����Զ�ʵ������Ӱ�죬��G����
H���ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ������Һ�����ȡ��ֵ����õ�NaOH��Һ��Ũ�Ƚ�ƫ��H��ȷ��
�ʴ�Ϊ��ABDH��
���� ���⿼�������ʺ����IJⶨ����Ҫ��������Һ�����ơ�����к͵ζ��IJ��������ݴ��������ʺ����ļ��㡢�������ȣ���Ŀ�Ѷ��еȣ������к͵ζ���ԭ���ǽ���Ĺؼ���


 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KClO3�ڷ�Ӧ�еõ����� | |
| B�� | ClO2���ȵĻ��ϼ�Ϊ+4�� | |
| C�� | �ڷ�Ӧ��H2C2O4�ǻ�ԭ�� | |
| D�� | 1 mol KClO3�μӷ�Ӧ��2mol����ת�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������м��ᣬ����Һ��pH ������2����Һ�Ի�ɫ��CrO42-����Ũ������ | |
| B�� | ������е���Һ�������ɫ����2v��CrO${\;}_{4}^{2-}$��=v��Cr2O72-��ʱ��˵����Ӧ2CrO42-����ɫ��+2H+?Cr2O72-����ɫ��+H2O �ﵽƽ��״̬ | |
| C�� | ������У���Ҫ��ԭ1 mol Cr2O${\;}_{7}^{2-}$���ӣ���Ҫ6 mol��NH4��2Fe��SO4��2•6H2O | |
| D�� | ������У�������Һ��pH ������4 ʱ������Ϊ��ˮ�еĸ�Ԫ���ѻ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.8��10-13 mol•L-1 | B�� | 7.3��10-13 mol•L-1 | ||
| C�� | 2.3 mol•L-1 | D�� | 3.7 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ksp��CaF2�����¶Ⱥ�Ũ�ȵı仯���仯 | |
| B�� | ��1 L0.2 mol•L-1 HF��Һ�м���1 L 0.2 mol•L-1 CaCl2��Һ��û�г������� | |
| C�� | AgCl������ˮ������ת��ΪAgI | |
| D�� | ����AgCl����NaI��Һ�п�ʼת��ΪAgI��NaIŨ�ȱ��벻����$\frac{1}{\sqrt{1.8}}$��10-11 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
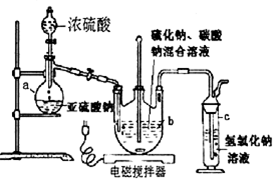
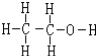 ��
��| ʵ����� | 1 | 2 | 3 |
| Na2S2O3��Һ�����mL�� | 19.98 | 20.02 | 21.18 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
| H2C2O4 | ��ɫ���� | K1=5.9��10-2��K2=6.4��10-5��������ˮ���Ҵ� |
| Na2C2O4 | ��ɫ���� | ����ˮ��pH=7.2���������Ҵ� |
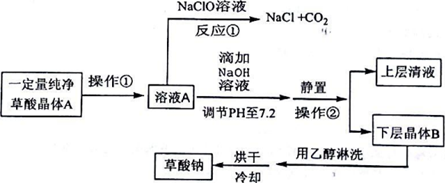
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com