
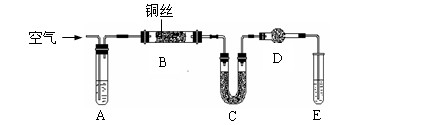
| | ʵ��Ŀ�� | ʵ����� |
| A | ʵ�����Ʊ������� | ������Ũ�����Ϻ���� |
| B | ��ȥ��������Һ�������Ȼ������� | �����Ȼ������ʵ���������Һ�м�����������������Һ������ |
| C | ���������鷢����ȥ��Ӧ�IJ��� | ��ʢ��������������Թ��У��ȼ�������������Һ�����ȣ��ٵ����������ữ ����������Һ ����������Һ |
| D | Ũ�����������ǿ��ϡ���� | ��ʢ��������Ũ���ᡢϡ�������֧�Թ��У��ֱ�����С��ͬ��ͭƬ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
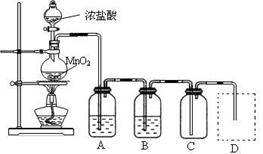
| ʵ�鷽�� | ʵ������ | ���� |
| ����Һ�м� KSCN��Һ | | ������������FeCl3 |
| ��a��Һ�еμ� ����KMnO4��Һ | KMnO4��Һ��ɫ ����ɫ | ���������в���______ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A���ü�ʽ�ζ���ȡ����ҺNaOH��Һ������ƿ���μ�2��3�η�ָ̪ʾ���� |
| B���ô����NaOH��Һ��ϴ��ʽ�ζ��ܡ� |
| C���ѵζ���������ˮϴ���� |
| D��ȡ����ʽ�ζ��ܣ��ñ���������Һ��ϴ���ٽ���������ע����ʽ�ζ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
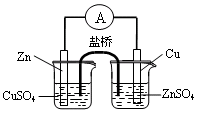
| A����ʪ���pH��ֽ�ⶨϡ�����pH |
| B������ͼ1����п��ͭԭ��� |
| C������ͼ2�����й�ʵ��������֪���ԣ�CH3COOH>H2CO3>C6H5OH |
| D������ͼ3��֤�����鷢����ȥ��Ӧ����ϩ�� |
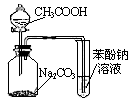
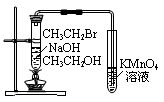
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
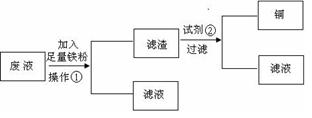
 ����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ�����
����д���������ĸ���ϵʽ�������жϣ�����ȷ�Ĺ�ϵʽ����� �����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ��
�����ڴ���ĺ���д����ȷ�Ĺ�ϵʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
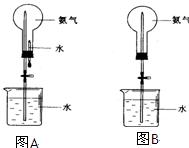
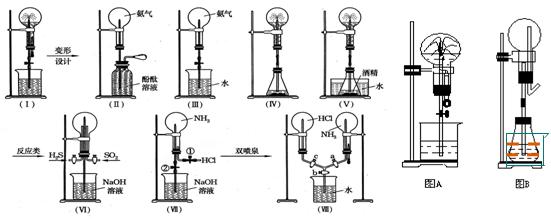
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��

| A����ʵ��֮�����ܹ�������Ȫ����Ϊ������������ˮ��������ƿ�������ѹǿ�� |
| B������ƿ�İ����л�����������������Ȫʵ����ˮ���ܳ�����ƿ |
| C����ʵ������NH3����HCl��Ҳ�ܲ�����ɫ����Ȫ |
| D��ʵ���������ƿ�а�ˮ�����ʵ���Ũ��Ϊ1/22.4 mol��L��1�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
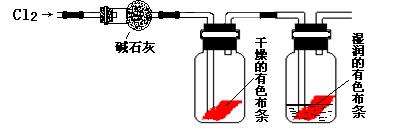
 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com