
��֪�����������ķе�Ϊ213�棨�ڴ��¶�����ˮ���Ҵ��ͻ�������7��0%��17��0%��76��0%�ı�����Ϊ�����ݳ�������ش�����ʵ�����Ʊ��������������й����⣺
��1������������ƿ�м��뱽���ᡢŨ���ᡢ�������Ҵ�����ʯ�����������ƿ�м��뻷���飬װ�Ϸ�ˮ���Ļ�����ȴ�ܡ�ʵ����ʹ�÷�ˮ����Ŀ���ǣ��ӻ�ѧƽ��ԭ��������_______________��
��2���������Ȼ���������ˮ���²�Һ�岻�����ֹ࣬ͣ���ȣ��ų���ˮ����Һ�壬��ˮ����Һ���������Ҫ�ɷ���____________��
��3����Բ����ƿ�еIJ�Һ����ʢ����ˮ����ƿ�У���_____________��Һ�к��������Է�Һ���ֳ��ֲ�Ʒ��ˮ��������____________����ʵ��������ƣ����Ѳ���ֲ�Ʒ�ϲ����ô�ˮϴ�л������Σ����Ѳ���ˮ�����־����Ѳ���Ͽڵ���һ���������ƿ��
��4����������������С����ˮ�Ȼ��Ƹ������ҡ����ƿ�����Ѳ���������Ѳ������һ�������Բ����ƿ������____________����ʵ��������ƣ���������__________������������������
��1�����뷴Ӧ���������ɵ�ˮ���ٽ�������Ӧ����������У���2��ˮ���Ҵ��ͻ����飻
��3������̼������Һ ��ȡ��4������ ����
���������������1����������Ҵ���������ӦΪ���淴Ӧ��ˮΪ�����ʵ����ʹ�÷�ˮ����Ŀ���Ƿ��뷴Ӧ���������ɵ�ˮ���ٽ�������Ӧ����������У���2���������Ϣˮ���Ҵ��ͻ�������7��0%��17��0%��76��0%�ı�����Ϊ�����ݳ�����ˮ����Һ���������Ҫ�ɷ���ˮ���Ҵ��ͻ����飻��3��Ǩ�ƽ̲������������Ʊ�ʵ���ñ���̼������Һ���к�����ͱ����ᣬ�ܽ��Ҵ����ұ����������ڱ���̼������Һ�е��ܽ�Ȳ������ڱ������������������ѣ�����������ȡˮ���в���ı�������������4�������뱽�������������Ҷ��߷е㲻ͬ�����ѵķе�ϵͣ��ʲ�������ķ��������߷��룬�е�͵������ȱ��������������������������
���㣺�������ʵ��Ʊ�����������롣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���嶡���Ա����ӣ�TBHQ����һ����ӱ��ʳƷ�����������Ʊ�ԭ�����£�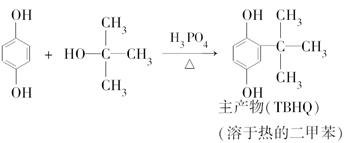
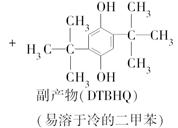
ʵ������е���Ҫ�������£�
����1����������ƿ�м���5.5 g�Ա����ӣ�5.0 mLŨ���ἰ20 mL���ױ���װ����ͼ��ʾ����������������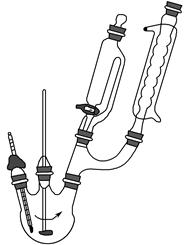
����2���������ȵ�100��110 �棬�����μ�7.5 mL�嶡����5 mL���ױ���ɵ���Һ��30��60 min�ڵ��ꡣ
����3�����µ�135��140 �棬���»���2.5 h��
����4������ӦҺ��ȴ��120 �棬ֱ����Ӧ��ɡ�
����5������ӦҺ�����ձ���������ˮϴ��������ƿ��ϴҺ�����ձ��С�
����6����ȴ�ᾧ�����ˣ�������Һ�еĶ��ױ������ᡣ
����7���øɼױ��ؽᾧ����ɫ����ˮϴ�ӡ����
��1��������ʵ���е�������________��
��2����ʵ���ж��ױ���������_________________________________ __________________________________________________��
��3������4�з�Ӧ��ɵı�־��_________________________��
��4������7��ɫʱ�����õ���ɫ����________��
��5���Ժϳɵõ��IJ�Ʒ����������Ҫ����Ҫ�ִ�����������__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ͨ������������������������ˮ�����ٷ�Һ�ŷŶԻ�������Ⱦ��ͬʱ����
K2Cr2O7��ʵ���ҶԺ�����Һ������Cr3+��Fe3+��K+��SO42����NO3��������Cr2O72���������������ù������£�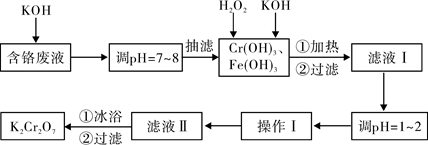
��֪����Cr(OH)3+OH��=CrO2��+2H2O��
��2CrO2��+3H2O2+2OH��=2CrO42��+4H2O��
��H2O2�����������¾��л�ԭ�ԣ��ܽ�+6��Cr��ԭΪ+3��Cr��
��1��ʵ��������KOHŨ��Ϊ6 mol��L��1������KOH��������250mL 6 mol��L��1 ��KOH��Һ�����ձ����������⣬�������õ��IJ��������� ��
��2�����ں�����Һ�к���������K2Cr2O7������ʱ���� ���沼��©�������˹���
��Ҫ��ʱ�۲�����ƿ��Һ��߶ȣ�����ﵽ֧�ܿ�λ��ʱӦ���еIJ���Ϊ ��
��3����Һ���ữǰ�����м��ȵ�Ŀ���� ����ԡ�����˺�Ӧ��������ˮϴ��K2Cr2O7����Ŀ���� ��
��4���±���������ʵ��ܽ�����ݣ�
| ���� | 0�� | 20�� | 40�� | 60�� | 80�� | 100�� |
| KCl | 28.0 | 34.2 | 40.1 | 45.8 | 51.3 | 56.3 |
| K2SO4 | 7.4 | 11.1 | 14.8 | 18.2 | 21.4 | 24.1 |
| K2Cr2O7 | 4.7 | 12.3 | 26.3 | 45.6 | 73.0 | 102.0 |
| KNO3 | 13.9 | 31.6 | 61.3 | 106 | 167 | 246.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
1�������������������ϣ�Ҳ�ɺϳ��������ϡ�ʵ�����Ʊ�1�����������Ĺ������£� 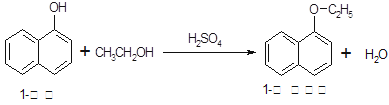
��֪����������ʵ���������
| ���� | ��Է� ������ | ״̬ | �۵�(��) | �е�(��) | �ܽ�� | |
| ˮ | �Ҵ� | |||||
| 1������ | 144 | ��ɫ���ɫ���νᾧ���ĩ | 96�� | 278�� | ����ˮ | �������Ҵ� |
| 1���������� | 172 | ��ɫҺ�� | 5��5�� | 267�� | ������ˮ | �������Ҵ� |
| �Ҵ� | 46 | ��ɫҺ�� | -114��1�� | 78��5�� | ����Ȼ��� | |

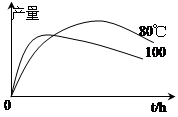
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��Ƴ������������ƹ��գ������ŷŵķ�ˮ�к��еľ綾CN�����ӣ����������ƹ�����������������Ƶķ�ˮʱ�����ڴ���TiO2�����£�����NaClO��CN������������CNO�����������������¼�����NaClO������N2��CO2������������Ա���ܱ�ϵͳ������ͼװ�ý���ʵ�飬��֤��������������Ч�ԣ����ⶨCN���������İٷ��ʡ���Ũ����CN�����ӵ���ˮ�����NaClO��Һ�Ļ��Һ��200mL������CN����Ũ��Ϊ0��05mol��L��1��������У�������Ƥ����һ��ʱ�����Ƥ���ͻ�����ʹ��Һȫ���������У��رջ�����
�ش��������⣺
�����з�Ӧ�����ӷ���ʽΪ ��
���������ɵ������N2��CO2�⣬����HCl��������Cl2�ȣ�����ʵ����ͨ���ⶨ������̼������ȷ����CN���Ĵ���Ч��������м���ij����Լ��� ������ĸ��
a������ʳ��ˮ b������NaHCO3��Һ c��ŨNaOH��Һ d��Ũ����
�Ƕ���ʵ���е������� ��װ�м�ʯ�ҵĸ���ܵ������� ��
������ʢ�к�Ca(OH)20��02mol��ʯ��ˮ����ʵ�������й�����0��82 g���������ʵ���в��CN���������İٷ��ʵ��� ����˵���ò��ֵ��ʵ�ʴ����İٷ������ƫ����ƫ�� ����Ҫ˵�����ܵ�ԭ�� ��
�������һ�������ȷ�ȵĽ��飨Ҫ�пɲ����ԣ�����ʹ������ù��ڸ��ӣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����̼��ƹ㷺Ӧ���������ϡ���ֽ����ѧ���ġ���ī��Ϳ�ϡ��ܷ⽺�뽺ճ������ҵ����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ơ�ijУѧ��ʵ��С�������ͼ��ʾװ�ã���ȡ�ò�Ʒ��D��װ��պϡ�������֬�ޣ�ͼ�мг�װ������ȥ��
��.��ѡ�õ�ҩƷ�У�
a��ʯ��ʯ b�������Ȼ�����Һ c��6 mol/L���� d���Ȼ�� e����������
��1��A���Ʊ�����ʱ������ҩƷ�ǣ�ѡ����ĸ��ţ� ��
��2��B��ʢ�б���̼��������Һ���������� ��
��3��д����ȡ�����Ļ�ѧ����ʽ ��
��4����ʵ������У���C��ͨ�����������Ⱥ�˳��ģ�Ӧ��ͨ������Ļ�ѧʽ ��
��5������D���ڴ��Ƿ��а����ݳ��ķ����� ��
��6��д��������̼��ƵĻ�ѧ����ʽ ��
��7����ʵ��������а����ݳ���Ӧѡ������ װ�û��գ�����ţ���
�������������Ȼ����Ʒ�к�������̼�����ơ�Ϊ�˲ⶨ�Ȼ�淋�������������ѧ��ʵ��С�������������ʵ�����̣�
�Իش�
��1�������Լ�A�Ļ�ѧʽΪ
��2��B����������
��3����Ʒ���Ȼ�淋���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ����ѧ��ѧ�г����ڻ����ķ�����ᴿ��װ�ã������װ�ûش����⣺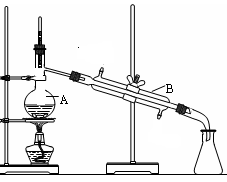
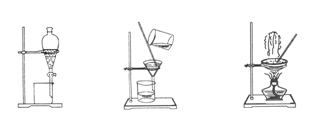
ͼ1 ͼ2 ͼ3 ͼ4
��1��װ��ͼ1��A��������________��B�������� ��A ��һ��Ҫ�������Ƭ���������� ��װ��ͼ4��ʢ��Һ������������ ��
��2��Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺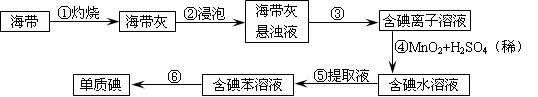
1)���պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������__________��������������ѡ�������������ñ����ĸ��д�ڿհ״�����
| A���ձ� | B������ | C�������� | D�������� E���ƾ��� F�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2������һ����Ȼ��ͭ������SiO2����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺
�ֳ�ȡ��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ��������½������գ�����Cu��Fe3O4��SO2���壬ʵ���ȡd����Һ�� ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00mL����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00mL����ش��������⣺
��1��������Ʒ���õ�����Ϊ_____���������ƽ��������ƽ����������Ʒ��ϸ���ٷ�Ӧ����Ŀ����_______ ��
��2��װ��a��c�����÷ֱ���____��____�����ţ���
a����ȥSO2����
b����ȥ�����е�ˮ����
c��������������
d�������ڹ۲��������
e����ȥ��Ӧ����������
��3��������Ӧ����������ͨһ��ʱ��Ŀ�������Ŀ����___________��
��4��ͨ�������֪���û�ͭ��Ĵ���Ϊ________��
��5��������ͼװ���������ʵ��װ��d��ͬ�����Դﵽʵ��Ŀ�ĵ���____������ţ���
��6������ԭװ��d�е���Һ��ΪBa(OH)2����õĻ�ͭ�����Ϊ��1%������ʵ���������ȷ�����ܵ�ԭ����Ҫ��_____________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com