
��20�֣�A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ�������AΪ���塢BΪ����ɫ���塢CΪ��ɫ���塣D��E��F��G��H��X��Ϊ���������X����������ɫ���壬��ˮ��Һ��һ������ǿ����Һ��EΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����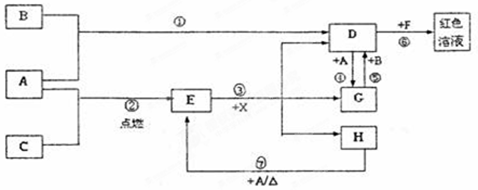
��1��д���������ʵĻ�ѧʽ��D X ��
��2���ڷ�Ӧ��~���У�������������ԭ��Ӧ���� �����ţ���
��3����Ӧ�����ӷ���ʽΪ�� ��
��4����G��Һ�м���NaOH��Һ�۲쵽�������� ��
��5����Ӧ�ߵĻ�ѧ����ʽΪ ���÷�Ӧ��ÿ����0.3mol��A����ת�Ƶ��� mol��
��6����ȥD��Һ�л��е�����G�ķ����ǣ�
��7����D��Һ���Ʊ���ˮD�ķ����ǣ�
��8���ֱ�д��D��Һ��С�մ���Һ��Ӧ�����ӷ���ʽ�ǣ�
 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2012?ʯ��ɽ��һģ��A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬CΪ���壮D��E��F��G��H��X��Y��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�壮����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ����
��2012?ʯ��ɽ��һģ��A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬BΪҺ�壬CΪ���壮D��E��F��G��H��X��Y��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�壮����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ����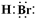
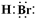
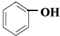

| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡ������ѧ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ������
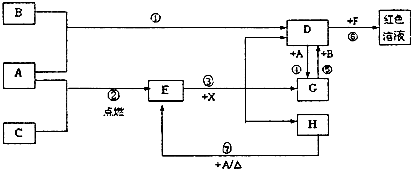
��11�֣�A��B��CΪ��ѧ�������ʣ�����һ��Ϊ ������ͨ�������AΪ
������ͨ�������AΪ ���塢BΪҺ�塢CΪ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����
���塢BΪҺ�塢CΪ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����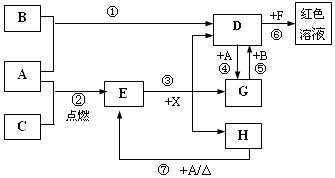
��1��д���������ʵĻ�ѧʽ�� D___________�� X____________��
��2���ڷ�Ӧ�١����У�������������ԭ��Ӧ����______�����ţ���
��3����Ӧ�����ӷ���ʽΪ��_____________________________________________________
��4����G��Һ�м���NaOH��Һ�۲쵽��������
��5����Ӧ�ߵĻ�ѧ����ʽΪ______________________________________________________��
�÷�Ӧ��ÿ����0��3 mol��A����ת�Ƶ���_______________mol��
��6��д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�ʡ������ѧ�ڵ������¿���ѧ�Ծ� ���ͣ������
��11�֣�A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ�������AΪ���塢BΪҺ�塢CΪ���塣D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�塣����֮���ת����ϵ��ͼ��ʾ������ijЩ��Ӧ�����Ͳ��ַ�Ӧ��������ȥ����
��1��д���������ʵĻ�ѧʽ�� D___________�� X____________��
��2���ڷ�Ӧ�١����У�������������ԭ��Ӧ����______�����ţ���
��3����Ӧ�����ӷ���ʽΪ��_____________________________________________________
��4����G��Һ�м���NaOH��Һ�۲쵽��������
��5����Ӧ�ߵĻ�ѧ����ʽΪ______________________________________________________��
�÷�Ӧ��ÿ����0��3 mol��A����ת�Ƶ���_______________mol��
��6��д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽΪ________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���½���³ľ����и������Ĵ��¿���ѧ�Ծ� ���ͣ������
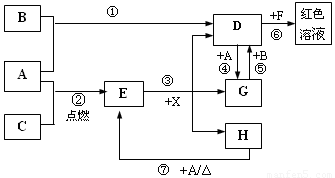
��11�֣�A��B��CΪ��ѧ�������ʣ�����һ��Ϊ������ͨ������£�AΪ���壬B��CΪ���壮D��E��F��G��H��X��Ϊ���������X��һ������ǿ�ᡢEΪ��ɫ���壬H�ڳ�����ΪҺ�壮����֮���ת����ϵ��ͼ��ʾ(����ijЩ��Ӧ�����Ͳ��ַ�Ӧ������ȥ)��

(1)д����ѧʽ��A_________��E__________��X__________.
(2)�ڷ�Ӧ�١����У�������������ԭ��Ӧ����________(����)��
(3)��Ӧ�����ӷ���ʽΪ��_________________________________________________.
(4)��Ӧ�ߵĻ�ѧ����ʽΪ__________________________________________________��
�÷�Ӧ��ÿ����0.3 mol��A����ת�Ƶ���________ mol.
(5)д��D����Һ��С�մ���Һ��Ӧ�����ӷ���ʽ��_______________________________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com