
��16�֣�X��Y��Z��W�dz��������ֶ�����Ԫ�أ���ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�����������Ų�ʽΪnsnnpn |
| Y | Y�ǿ����к�����ߵ�Ԫ�� |
| Z | Z�ǵؿ��к�����ߵĽ���Ԫ�� |
| W | W�ĵ����dz����İ뵼����� |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�������H��He��N��Na��Mg��Si��Ԫ�أ�������δ������Դ���⡣
��1��3He�Ǹ�Ч����ԭ�ϣ���ԭ�Ӻ���������Ϊ_____________��
��2��Na��ԭ�ӽṹʾ��ͼΪ______��Na����������ȫȼ�����ò���ĵ���ʽΪ_______��
��3��MgCl2�ڹ�ҵ��Ӧ�ù㷺������MgO�Ʊ���
��MgO���۵��BeO���۵�________����ߡ��͡���
��������ij��ʯ�������õ���MgO�к�������SiO2����ȥSiO2�����ӷ���ʽΪ______��SiO2�ľ�������Ϊ________��
��MgO��̿�ۺ�������һ�������·�Ӧ���Ʊ�MgCl2����β����������NaOH��Һ��ȫ���գ������ɵ���Ϊ______��д��ѧʽ����
��4�������к��зḻ��3He��������������1 kg3Heͬʱ�ɵ�6000kgH2��700kgN2����������H2��N2Ϊԭ�Ͼ�һϵ�з�Ӧ�����Ƶ�̼�����___kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�A��B��C��D��EΪԭ���������������Ԫ�أ�����ֻ��E�����ڶ����ڣ������Ϣ���±���
| Ԫ�� | A | B | C | D | E |
| ��� ��Ϣ | �������������۴�����Ϊ2 | ��Ԫ��C���γ����Ӹ�����Ϊ2��1��1��1�Ļ����� | ����������ͨ��������ú���� | DԪ�ؿ��γ��������������һ�����γ��������Ҫ�ɷ� | �䵥������;��㷺�Ľ���������ȱ�ٸ�Ԫ����ƶѪ֢ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(12��) ���Ʒ��ǿ�ѧѧϰ����Ҫ����֮һ
���������ƽ�����ȷ����
| | ��ȶ��� | ���� |
| A | Cl2+H2O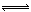 HCl+HClO HCl+HClO | I2+H2O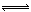 HI+HIO HI+HIO |
| B | C+2CuO ="==" 2Cu+CO2�������������ȣ� | C+SiO2 ="==" Si+ CO2�������������ȣ� |
| C | Na2O+H2O ="=" 2NaOH | CuO+H2O ="=" Cu(OH)2 |
| D | Ca(ClO)2+CO2+H2O="=" CaCO3��+2HClO | Ca(ClO)2+SO2+H2O="=" CaSO3��+2HClO |
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵�(��) | ��218.4 | 113 | | 450 |
| ���ʷе�(��) | ��183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | ��2 | ��2��+4��+6 | ��2��+4��+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��10�֣�Q��W��X��Y��Z��ԭ��������������Ķ�����Ԫ�أ�X��Y�ǽ���Ԫ�أ�Q��W��Z�Ƿǽ���Ԫ�ء�����Ԫ�غ˵����֮��Ϊ55����Ӧԭ������������֮��Ϊ21��W��Z������������ͬ����Z�ĺ˵������W��2����
��1��Q�����ڱ���λ�ڵ� ���� �塣
��2��X��Y���Ե�����������Ӧ��ˮ������Է�����Ӧ�����κ�ˮ����д���÷�Ӧ�����ӷ���ʽ�� ��
��3��X��������W������ȼ�տ����ɻ�����R��R�ĵ���ʽ ___�������������еĻ�ѧ��������Ϊ ��
��4��Z���⻯����W���⻯�����Ӧ����Z���ʺ�ˮ��д���仯ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ--ѡ��3���ʽṹ�����ʡ���15�֣�
ͭ���������ճ������г����Ľ��������ǵĵ��ʼ��仯�����ڿ�ѧ�о���ũҵ�����о��й㷺��;��
��ش��������⣺
��1����ϸͭ�ۿ�����������ϡ������ȣ����Ʊ��������£�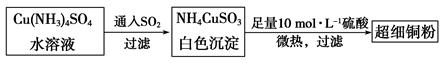
��Cu2+�ļ۵����Ų�ͼ ; NH4CuSO3��N��O��S����Ԫ�صĵ�һ�������ɴ�С˳��Ϊ_______________________(��Ԫ�ط���)��
��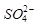 �Ŀռ乹��Ϊ_____________��
�Ŀռ乹��Ϊ_____________��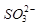 ��������ԭ�ӵ��ӻ���ʽΪ ��
��������ԭ�ӵ��ӻ���ʽΪ ��
��2����д����Cu(NH3)4SO4ˮ��Һ��ͨ��SO2ʱ������Ӧ�����ӷ���ʽ�� ��
��3��ijѧ����CuSO4��Һ�м���������ˮ������ɫ�������������������ˮ�����ܽ⣬�õ�����ɫ����Һ����������Һ�м���һ�����Ҵ�������[Cu(NH3)4]SO4��H2O���塣
������˵����ȷ����
a��������������ˮ������ΪNH3���Ӻ�H2O����֮���γ�3�ֲ�ͬ�����
b��NH3���Ӻ�H2O���ӣ����ӿռ乹�Ͳ�ͬ���������ӵļ���С��ˮ���ӵļ���
c��Cu(NH3)4SO4�����еĻ�ѧ�������Ӽ������Թ��ۼ�����λ��
d��Cu(NH3)4SO4���Ԫ���е縺�������ǵ�Ԫ��
������ͼ����Ҵ������������ԭ�� ��
��4��Cu����Ķѻ���ʽ��ͼ��ʾ����Cuԭ�Ӱ뾶Ϊr,
������Cuԭ�ӵ���λ��Ϊ_______������Ŀռ�������
Ϊ �� 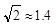 ,��ʽ������������
,��ʽ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��24�֣��±���Ԫ�����ڱ���һ���֣���Ա��Т١�����Ԫ�أ���д���пհף�
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | �� | |
| 3 | �� | �� | �� | | | | �� | |
| 4 | �� | �� | | | | | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�(��д��Ų��÷�)��
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | | �� | | �� | �� | �� |
| 4 | �� | | | | | | �� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±���Ԫ�����ڱ�����Ԫ�ص�һ���֣�������Ԫ��X����������ϼ��ǣ�5��Y�ĵ���
���ڿ�����ȼ�ա�
| W | X | Y |
| | | Z |
| ��� | �����Ʋ� | ��ѧ����ʽ |
| ʾ�� | ������ | H2ZO3��4HI=Z����2I2��3H2O |
| 1 | | |
| 2 | | |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com