
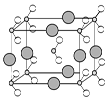


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
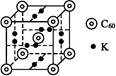
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����X��Y��ɵĻ�����ķе���ܸ�����X��Z��ɵĻ�����ķе� |
| B��X��Z��R����Ԫ���е�����������ϳɵģ�1��1�ͣ������о����й��ۼ� |
| C����ѹ�����£��е㣺X2Z2��XM �������Ӱ뾶��Z��M��R��W |
| D��R 3WM6�������Ϊ��ҵ�������W2Y3��W����ʱ�����ۼ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
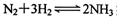 ʵ�ִ�������⡣����˵����ȷ���� ��
ʵ�ִ�������⡣����˵����ȷ���� ��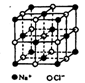
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2NH3ʵ�ִ�������⡣����˵����ȷ����________(����ѡ��)��
2NH3ʵ�ִ�������⡣����˵����ȷ����________(����ѡ��)��| A��NH3������Nԭ�Ӳ���sp3�ӻ� |
| B����ͬѹǿʱ��NH3�е��PH3�� |
| C��[Cu (NH3)4]2�������У�Nԭ������λԭ�� |
| D��CN���ĵ���ʽΪ[:C����N:]�� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| Ԫ�� | �����Ϣ |
| X | XԪ���γɵ�һ��ͬ������������Ȼ������Ӳ�����ĵ��� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬��������ڻ�ҩ |
| Z | Z�Ļ�̬ԭ�Ӻ�����3���ܼ����е��ӣ�����3�������� |
| W | WԪ���γɵ�˫ԭ�ӷ��ӣ�������Ϊ����ɫ���壬һ�ֳ�����ҵԭ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com