
��֪B�dz����������ʣ�EΪ�����ǽ������ʣ�H������Ϊ��ɫҺ�壬C��Ũ��Һ�ڼ���ʱ����D��Ӧ���������п�ͼ��ʾ���Իش�
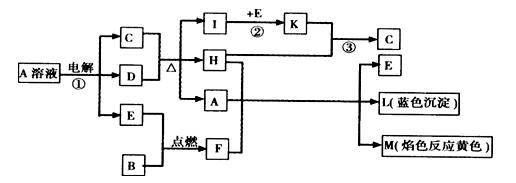
(1)д����ѧʽ��A____________��E______________��L______________��
(2)��Ӧ�ٵ����ӷ���ʽ��___________________________________��
(3)��Ӧ�ڣ���ҵ�ϲ�ȡ�ķ�Ӧ������________________________��
(4)��Ӧ�ۣ���ҵ�ϲ�ȡ�IJ�������Kֱ����H��Ӧ��ԭ����__________________��
(5)ÿ����1 mol K����Ӧ�ų�98.3 kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ��
___________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
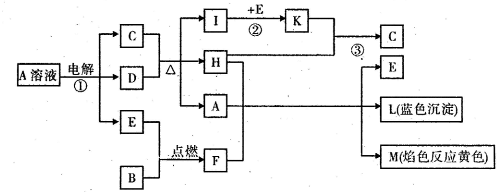
��֪B�dz����������ʣ�EΪ�����ǽ������ʣ�H������Ϊ��ɫҺ�壬C��Ũ��Һ�ڼ���ʱ����D��Ӧ���������п�ͼ��ʾ���Իش�
(1)д����ѧʽ��A____________��E______________��L_______________��
(2)��Ӧ�ٵ����ӷ���ʽ��__________________________________________________��
(3)��Ӧ�ڣ���ҵ�ϲ�ȡ�ķ�Ӧ������________________________________��
(4)��Ӧ�ۣ���ҵ�ϲ�ȡ�IJ�������Kֱ����H��Ӧ��ԭ����_______________
____________________________________________________��
(5)ÿ����1 mol K����Ӧ�ų�98.3 kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ��
______________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���Ĵ�ʡ�Թ��и�����ѧ�ڵ�һ������Կ����������⣨��ѧ���֣� ���ͣ������
(14��)��֪B�dz����������ʣ�EΪ�����ǽ�������,H������Ϊ��ɫҺ�壬C��Ũ��Һ�ڼ���ʱ����D��Ӧ���������п�ͼ��ʾ���Իش�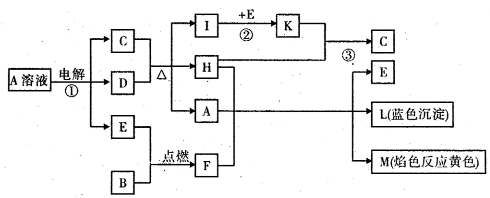
��д����ѧʽ��A______ �� E______�� L______
(2)��Ӧ�ٵ����ӷ���ʽ��________________________
�Ƿ�Ӧ�ڣ���ҵ���ȡ�ķ�Ӧ������________________________
(4)��Ӧ�ۣ���ҵ���ȡ�IJ�������Kֱ����H��Ӧ��ԭ����__________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ĵ�ʡ������ѧ��3���¿������ۣ���ѧ���� ���ͣ������
��֪B�dz����������ʣ�EΪ�����ǽ������ʣ�H������Ϊ��ɫҺ�壬C��Ũ��Һ�ڼ���ʱ����D��Ӧ���������п�ͼ��ʾ���Իش�

(1)д����ѧʽ��A____________��E______________��L______________��
(2)��Ӧ�ٵ����ӷ���ʽ��___________________________________��
(3)��Ӧ�ڣ���ҵ�ϲ�ȡ�ķ�Ӧ������________________________��
(4)��Ӧ�ۣ���ҵ�ϲ�ȡ�IJ�������Kֱ����H��Ӧ��ԭ����__________________��
(5)ÿ����1 mol K����Ӧ�ų�98.3 kJ�������÷�Ӧ���Ȼ�ѧ����ʽΪ��
___________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com