
| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |
| Ũ���� |
| 170�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ʡ��֣һ�и߶���ѧ�ھ�������ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
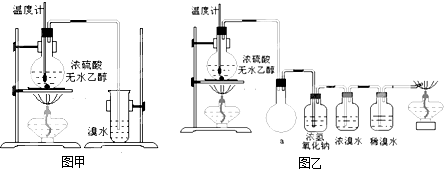
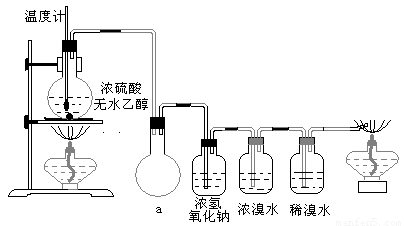
(14��)Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ļӳɷ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���������ʵ�顣���¶�����170������ʱ���д����������������������ͨ����ˮ�У���ˮ����ɫѸ����ȥ����ͬѧ��Ϊ�ﵽ��ʵ��Ŀ�ġ�
��ͬѧ��ϸ�����˼�ͬѧ������ʵ����̣����ֵ��¶�����100������ʱ����ɫҺ�忪ʼ��ɫ����160������ʱ�����Һȫ�ʺ�ɫ����170�泬�������������ٶ����Լӿ죬���ɵ������д̼�����ζ���ɴ����Ƴ���������������Ӧ�����ʣ�����Ӱ����ϩ�ļ���������ȥ���ݴ˻ش��������⣺
(1)д����ͬѧʵ����������Ӧ�Ļ�ѧ����ʽ��____________________________________��
____________________________________��
(2)��ͬѧ�۲쵽�ĺ�ɫ������__________���̼���������__________����ͬѧ��Ϊ�̼�������Ĵ��ھͲ�����Ϊ��ˮ��ɫ����ϩ�ļӳɷ�Ӧ��ɵġ�
ԭ����(�û�ѧ���̱�ʾ)��__________________��
(3)��ͬѧ���ݼ���ͬѧ�ķ�������Ϊ��������CO��CO2�������������Ϊ֤��CO���ڣ�����������¹���(�ù��̿ɰ�ʵ���в������л��������)������������徭��ȼ����ɫ���棬ȷ����һ����̼��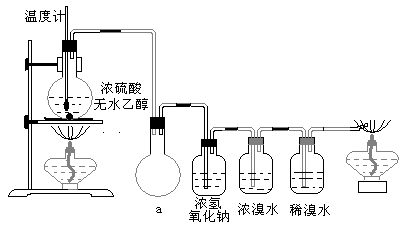
�����װ��a��������_____________________________________________
��Ũ��ˮ��������_________________________________________________��
ϡ��ˮ��������___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ԭ���и߶���ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(14��)Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ļӳɷ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���������ʵ�顣���¶�����170������ʱ���д����������������������ͨ����ˮ�У���ˮ����ɫѸ����ȥ����ͬѧ��Ϊ�ﵽ��ʵ��Ŀ�ġ�
��ͬѧ��ϸ�����˼�ͬѧ������ʵ����̣����ֵ��¶�����100������ʱ����ɫҺ�忪ʼ��ɫ����160������ʱ�����Һȫ�ʺ�ɫ����170�泬�������������ٶ����Լӿ죬���ɵ������д̼�����ζ���ɴ����Ƴ���������������Ӧ�����ʣ�����Ӱ����ϩ�ļ���������ȥ���ݴ˻ش��������⣺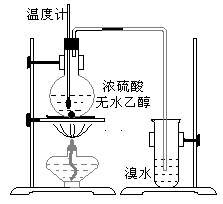
(1)д����ͬѧʵ����������Ӧ�Ļ�ѧ����ʽ��
___________________________��__________________________��
(2)��ͬѧ�۲쵽�ĺ�ɫ������_____________���̼���������_____________����ͬѧ��Ϊ�̼�������Ĵ��ھͲ�����Ϊ��ˮ��ɫ����ϩ�ļӳɷ�Ӧ��ɵġ�ԭ����(�û�ѧ���̱�ʾ)��_____________________��[��Դ:ѧ|��|��]
(3)��ͬѧ���ݼ���ͬѧ�ķ�������Ϊ��������CO��CO2�������������Ϊ֤��CO���ڣ�����������¹���(�ù��̿ɰ�ʵ���в������л��������)������������徭��ȼ����ɫ���棬ȷ����һ����̼��
�����װ��a��������____________________
��Ũ��ˮ��������_______________________��ϡ��ˮ��������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ�ھ�������ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(14��)Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ļӳɷ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���������ʵ�顣���¶�����170������ʱ���д����������������������ͨ����ˮ�У���ˮ����ɫѸ����ȥ����ͬѧ��Ϊ�ﵽ��ʵ��Ŀ�ġ�
��ͬѧ��ϸ�����˼�ͬѧ������ʵ����̣����ֵ��¶�����100������ʱ����ɫҺ�忪ʼ��ɫ����160������ʱ�����Һȫ�ʺ�ɫ����170�泬�������������ٶ����Լӿ죬���ɵ������д̼�����ζ���ɴ����Ƴ���������������Ӧ�����ʣ�����Ӱ����ϩ�ļ���������ȥ���ݴ˻ش��������⣺
(1)д����ͬѧʵ����������Ӧ�Ļ�ѧ����ʽ��____________________________________��
____________________________________��
(2)��ͬѧ�۲쵽�ĺ�ɫ������__________���̼���������__________����ͬѧ��Ϊ�̼�������Ĵ��ھͲ�����Ϊ��ˮ��ɫ����ϩ�ļӳɷ�Ӧ��ɵġ�
ԭ����(�û�ѧ���̱�ʾ)��__________________��
(3)��ͬѧ���ݼ���ͬѧ�ķ�������Ϊ��������CO��CO2�������������Ϊ֤��CO���ڣ�����������¹���(�ù��̿ɰ�ʵ���в������л��������)������������徭��ȼ����ɫ���棬ȷ����һ����̼��
�����װ��a��������_____________________________________________
��Ũ��ˮ��������_________________________________________________��
ϡ��ˮ��������___________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
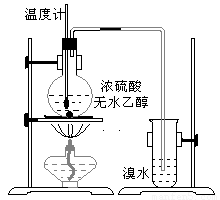
(14��)Ϊ̽��ʵ��������ϩ����ϩ����ˮ�ļӳɷ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã���������ʵ�顣���¶�����170������ʱ���д����������������������ͨ����ˮ�У���ˮ����ɫѸ����ȥ����ͬѧ��Ϊ�ﵽ��ʵ��Ŀ�ġ�
��ͬѧ��ϸ�����˼�ͬѧ������ʵ����̣����ֵ��¶�����100������ʱ����ɫҺ�忪ʼ��ɫ����160������ʱ�����Һȫ�ʺ�ɫ����170�泬�������������ٶ����Լӿ죬���ɵ������д̼�����ζ���ɴ����Ƴ���������������Ӧ�����ʣ�����Ӱ����ϩ�ļ���������ȥ���ݴ˻ش��������⣺

(1)д����ͬѧʵ����������Ӧ�Ļ�ѧ����ʽ��
___________________________��__________________________��
(2)��ͬѧ�۲쵽�ĺ�ɫ������_____________���̼���������_____________����ͬѧ��Ϊ�̼�������Ĵ��ھͲ�����Ϊ��ˮ��ɫ����ϩ�ļӳɷ�Ӧ��ɵġ�ԭ����(�û�ѧ���̱�ʾ)��_____________________��[��Դ:ѧ|��|��]
(3)��ͬѧ���ݼ���ͬѧ�ķ�������Ϊ��������CO��CO2�������������Ϊ֤��CO���ڣ�����������¹���(�ù��̿ɰ�ʵ���в������л��������)������������徭��ȼ����ɫ���棬ȷ����һ����̼��

�����װ��a��������____________________
��Ũ��ˮ��������_______________________��ϡ��ˮ��������____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com