

 +NaOH��
+NaOH�� +CH3OH��
+CH3OH�� ���������ͨ����ȥ��Ӧ���ɻ�����I�Ļ�ѧ����ʽΪ
���������ͨ����ȥ��Ӧ���ɻ�����I�Ļ�ѧ����ʽΪ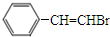 +NaOH$��_{��}^{��}$
+NaOH$��_{��}^{��}$ +NaBr+H2O��ע����Ӧ��������
+NaBr+H2O��ע����Ӧ��������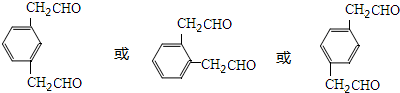
 ����Ľṹ��ʽΪ
����Ľṹ��ʽΪ
 ��
�� ���� I�����ӳɷ�Ӧ����II��III���巢���ӳɷ�Ӧ����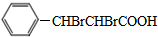 ����III�ṹ��ʽΪ
����III�ṹ��ʽΪ ��
��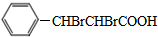 ��̼���Ʒ�Ӧ����
��̼���Ʒ�Ӧ����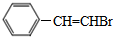 ��
��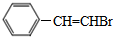 ������ȥ��Ӧ���ɱ���Ȳ��
������ȥ��Ӧ���ɱ���Ȳ��
��1��������I��ϵͳ����������Ϊ����Ȳ��
��2�����ݽṹ��ʽȷ������ʽ�����������NaOH��Һ����ȫˮ�⣬���ݷ�Ӧ�����������д��Ӧ����ʽ��
��3��������III�Ľṹ��ʽΪ �����ݷ�Ӧ�����������д��Ӧ����ʽ��
�����ݷ�Ӧ�����������д��Ӧ����ʽ��
��4����������ǻ�������ͬ���칹�壬������������ȡ�������ܷ���������Ӧ��˵����ȩ�������ĺ˴Ź������׳��������������壬�����֮��Ϊ1��2��˵������ȡ�����ϵ���ԭ����֮��Ϊ1��2���ݴ�д�����Ľṹ��ʽ��
��5�����ݾۺ���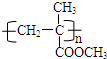 ��֪��������Ϊ�Ӿ۲���ݴ˿�д�����壬��Ӧ��ΪȲ��CO���״���Ӧ����ϡ����
��֪��������Ϊ�Ӿ۲���ݴ˿�д�����壬��Ӧ��ΪȲ��CO���״���Ӧ����ϡ����
��� �⣺I�����ӳɷ�Ӧ����II��III���巢���ӳɷ�Ӧ����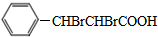 ����III�ṹ��ʽΪ
����III�ṹ��ʽΪ ��
��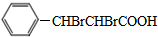 ��̼���Ʒ�Ӧ����
��̼���Ʒ�Ӧ����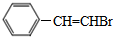 ��
��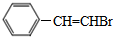 ������ȥ��Ӧ���ɱ���Ȳ��
������ȥ��Ӧ���ɱ���Ȳ��
��1��������I��ϵͳ����������Ϊ����Ȳ���ʴ�Ϊ������Ȳ��
��2�����ݻ������Ľṹ��ʽ��֪�����ʽΪC10H10O2��������������������ڼ����������ܷ���ˮ�ⷴӦ���������ƺʹ�����ѧ����ʽΪ +NaOH��
+NaOH�� +CH3OH��
+CH3OH��
�ʴ�Ϊ��C10H10O2�� +NaOH��
+NaOH�� +CH3OH��
+CH3OH��
��3�����ݻ����������ӳɵ�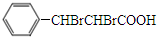 ����֪�������Ϊ
����֪�������Ϊ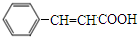 ���������ͨ����ȥ��Ӧ���ɻ�����IΪ
���������ͨ����ȥ��Ӧ���ɻ�����IΪ ����Ӧ�Ļ�ѧ����ʽΪ
����Ӧ�Ļ�ѧ����ʽΪ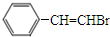 +NaOH$��_{��}^{��}$
+NaOH$��_{��}^{��}$ +NaBr+H2O��
+NaBr+H2O��
�ʴ�Ϊ��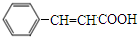 ��
��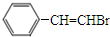 +NaOH$��_{��}^{��}$
+NaOH$��_{��}^{��}$ +NaBr+H2O��
+NaBr+H2O��
��4����������ǻ�������ͬ���칹�壬������������ȡ�������ܷ���������Ӧ��˵����ȩ�������ĺ˴Ź������׳��������������壬�����֮��Ϊ1��2��˵������ȡ�����ϵ���ԭ����֮��Ϊ1��2���������������Ģ��Ľṹ��ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5�����ݾۺ���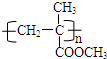 ��֪��������Ϊ�Ӿ۲���������ĵ���Ϊ
��֪��������Ϊ�Ӿ۲���������ĵ���Ϊ ����Ȳ����ʵ�ԭ�Ͽ��Ժϳɸõ���Ļ�ѧ����ʽΪCH3C��CH+CO+CH3OH$\stackrel{һ��������}{��}$
����Ȳ����ʵ�ԭ�Ͽ��Ժϳɸõ���Ļ�ѧ����ʽΪCH3C��CH+CO+CH3OH$\stackrel{һ��������}{��}$ ��
��
�ʴ�Ϊ�� ��CH3C��CH+CO+CH3OH$\stackrel{һ��������}{��}$
��CH3C��CH+CO+CH3OH$\stackrel{һ��������}{��}$ ��
��
���� ���⿼���л���ṹ�����ʼ��л����ƶϣ�Ϊ��Ƶ���㣬��ȷ�����ż������ʹ�ϵ�ǽⱾ��ؼ�������ݷ�Ӧ������Ӧ���������ṹ��ʽ�����ƶϣ���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������ͨ��Ʒ����Һ����Һ�Ժ�ɫ�����Ⱥ��Ϊ��ɫ | |
| B�� | ����Һ�м���������ˮ���ٵμ�KSCN��Һ������Һ��죬��֤��ԭ��Һ��һ������Fe2+ | |
| C�� | ��ʪ��ĺ�ֽ���Ž�ʢ�������ļ���ƿ�У���ֽ����ɫ | |
| D�� | �����Ը��������Һ�еμ�Na2SO3��Һ����Һ�����Ա仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ȫ�� | B�� | �٢ۢܢ� | C�� | �ڢۢܢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ά�ء� ���� | B�� | ��ѿ�ǡ� �ȵ��� | ||
| C�� | ����ϩ������ȩ��֬ | D�� | ���� �������ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ij��Һ�м���ϡ�����ữ���ٵ���BaCl2��Һ��������ɫ��������ԭ��Һ��һ����SO42- | |
| B�� | ��ij��Һ�м���ϡ�����ữ���ٵ���AgNO3��Һ��������ɫ��������ԭ��Һ��һ����Cl- | |
| C�� | ��ij��Һ�м���̼������Һ��������ɫ�������ٵ���ϡ���ᣬ�����ܽ⣬��ԭ��Һ��һ����Ca2+ | |
| D�� | �ù��IJ�˿պȡij��ɫ��Һ���ھƾ�������������ʱ�۲쵽��ɫ���棬��ԭ��Һ��һ����Na+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 Ǧ���صĵ���ܷ�ӦʽΪ��
Ǧ���صĵ���ܷ�ӦʽΪ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com