
����Ŀ��B��N��Ti��Fe������Ҫ�IJ���Ԫ�أ��䵥�ʼ�����������������ж��й㷺��Ӧ�á�
��1����̬Fe2���ĵ����Ų�ʽΪ_____��Tiԭ�Ӻ����________���˶�״̬��ͬ�ĵ��ӡ�
��2��BH3������NH3���ӵĿռ�ṹ�ֱ�Ϊ_________��BH3��NH3��Ӧ���ɵ�BH3��NH3�����к��еĻ�ѧ��������_______����BH3��NH3��Bԭ�ӵ��ӻ���ʽΪ________��
��3��N��Pͬ���塣��ѧ��Ŀǰ�ϳ���N4���ӣ��÷�����N��N��N���ļ���Ϊ________��N4�ֽ���ܲ���N2���ͷų������������Ʋ�����;___________��(д��һ�ּ���)
��4��NH3��Cu2�����γ�[Cu(NH3)4]2�������ӡ���֪NF3��NH3������ͬ�Ŀռ乹�ͣ���NF3������Cu2���γ������ӣ���ԭ����____��
��5������TiO2��һ��Ӧ�ù㷺�Ĵ����������һ��ʵ����ͼ��ʾ���������ҵķе����Ը��ڻ�����ף���Ҫԭ����______�����������в�ȡsp3�ӻ���ԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ________��
���𰸡�1s2 2s2 2p6 3s2 3p6 3d6 ��[Ar]3d6 22 ƽ���������Ρ������� ���ۼ�����λ�� sp3 60�� �������ƽ�����ըҩ(���������𰸾���) F �ĵ縺�Ա� N ��N��F �ɼ����Ӷ�ƫ�� F������ NF3 �е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ�� �������ҷ��Ӽ������� N>O>C
��������
(1)��Ϊ26��Ԫ�أ�ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+���ݴ���д��������Ų�ʽ�������ÿ�����ӵ��˶�״̬����ͬ��
(2)NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�������ռ乹�Ͳ�ͬ�����ݼ۲���ӶԻ�������ȷ��BF3�ķ��ӿռ乹�ͣ�
(3)N4������P4�ṹ���ƣ�Ϊ�������幹�ͣ�ÿ�����Ϊ�������Σ�N4�ֽ���ܲ���N2���ͷų������������ݴ��ж���;��
(4)NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�
(5)����Ĵ��ڵ��������۷е����ߣ��������������γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ��ݴ�����
(1)��ԭ��ʧȥ�����4s�ܼ�2�����ӣ��γ�Fe2+����������Ų�ʽΪ��1s22s22p63s23p63d6��[Ar]3d6��TiԪ��ԭ�Ӻ�����22�����ӣ�����ԭ�����˶�״̬��ͬ�ĵ��ӹ���22�֣��ʴ�Ϊ��1s22s22p63s23p63d6��[Ar]3d6��22��
(2)NH3�����е�ԭ�Ӻ��йµ��Ӷԣ�BF3������BԪ�ز����µ��Ӷԣ�BF3��Bԭ�Ӻ���3�������Ҳ����µ��Ӷԣ�����BF3Ϊƽ�������ι��ͣ�NH3��Nԭ�Ӻ���3��������1���µ��Ӷԣ�����NH3Ϊ�������ͣ�BF3NH3�����к��еĻ�ѧ�������й��ۼ�����λ������BF3NH3��Bԭ�ӵļ۲���Ӷ���Ϊ4+0=4���ӻ���ʽΪsp3���ʴ�Ϊ��ƽ���������Σ������ͣ����ۼ�����λ����sp3��
(3)N4������Nԭ���γ�3������������1�Թ¶Ե��ӣ��ӻ������ĿΪ4��Nԭ�Ӳ�ȡsp3�ӻ���ÿ����Ϊ�������Σ�N-N ���ļ���Ϊ60����N4�ֽ���ܲ���N2���ͷų����������������������ƽ�����ըҩ���ʴ�Ϊ��60�����������ƽ�����ըҩ��
(4)F�ĵ縺�Դ���NԪ�أ�NF3��N-F�ɼ����Ӷ�ƫ����Fԭ�ӣ�����NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ��Nԭ���ϵŶԵ�������ͭ�����γ������ӣ�����NF3������Cu2+�γ������ӣ��ʴ�Ϊ��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��F������NF3�е�ԭ�Ӻ˶���µ��ӶԵ�����������ǿ�������γ���λ����
(5)����Ĵ��ڻᵼ�������۷е����ߣ����к��з��Ӽ�������ײ�����������Ի��������۷е���ڼף��������������γ�sp3�ӻ���ԭ����C��N��OԪ�أ�ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե�һ������N��O��C���ʴ�Ϊ���������ҷ��Ӽ���������N��O��C��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ܱ������У����ȵ����ʵ�����NaHCO3��Na2O2�Ĺ��������ַ�Ӧ�������й���ʣ������( )
A. Na2CO3��Na2O2 B. Na2CO3��NaOH
C. NaOH��Na2O2 D. NaOH��Na2O2��Na2CO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ͼ���ʾ�������к�SiO2��Fe2O3��CeO2��FeO�����ʡ�ij����С���Դ˲�����ĩΪԭ�ϣ��Ƶ� Ce(OH)4��������立���Fe2(SO4) 3(NH4) 2SO424H2O������������£�

��֪�������������£�����ˮ��Һ����Ce3+��Ce4+������Ҫ������ʽ��Ce4+�н�ǿ�����ԣ���CeO2������ϡ���ᣬҲ������ NaOH��Һ��
�ش��������⣺
��1����Ӧ����H2O2��������___________��
��2����Ӧ�۵����ӷ���ʽ��________��
��3����֪�л��� HT �ܽ�Ce3+��ˮ��Һ����ȡ�������ù��̿ɱ�ʾΪ��2Ce3+ (ˮ��)+ 6HT���л��㣩![]() 2CeT3 (�л��㣩+ 6H+(ˮ��),��ƽ��ǶȽ��ͣ��� CeT3 (�л��㣩����H2SO4��ýϴ��ĺ�Ce3+��ˮ��Һ��ԭ����_______��
2CeT3 (�л��㣩+ 6H+(ˮ��),��ƽ��ǶȽ��ͣ��� CeT3 (�л��㣩����H2SO4��ýϴ��ĺ�Ce3+��ˮ��Һ��ԭ����_______��
��4��������立���Fe2(SO4) 3��(NH4) 2SO4��24H2O�ݹ㷺����ˮ�ľ����������侻ˮԭ�������ӷ���ʽ������___________��
��5���õζ����ⶨ�Ƶõ� Ce(OH)4 ��Ʒ���ȡ�
![]()
������FeSO4��Һ�ڿ�����¶��һ��ʱ����ٽ��еζ������ø�Ce(OH)4 ��Ʒ����������____�����ƫ����ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�Ϊ2 L�ĺ����ܱ������г���1 mol CO2(g)��3.5 mol H2(g)����һ�������·�����Ӧ��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H<0����Ӧ����8 minʱ�ﵽƽ��״̬�����n(CH3OH)=0.5mol���÷�Ӧ��ƽ�ⳣ��K���¶�T�Ĺ�ϵ��ͼ1��ʾ��CO2��ת������ͼ2��ʾ������˵���������
CH3OH(g)+H2O(g) ��H<0����Ӧ����8 minʱ�ﵽƽ��״̬�����n(CH3OH)=0.5mol���÷�Ӧ��ƽ�ⳣ��K���¶�T�Ĺ�ϵ��ͼ1��ʾ��CO2��ת������ͼ2��ʾ������˵���������
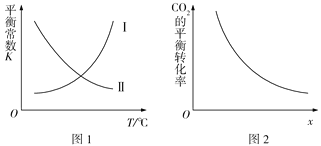
A. ��ͼ1�У����ߢ��ʾ�÷�Ӧ��ƽ�ⳣ��K���¶�T�Ĺ�ϵ
B. ���¶��£�ƽ�ⳣ��K=0.25
C. �������������£�ͼ2��x�ɱ�ʾ�¶Ȼ�ѹǿ��Ͷ�ϱ�c(CO2)/c(H2)
D. �ö�����̼�ϳɼ״�������̼��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯���������������о�����Ҫ�����á�
(1)�����ڻ��ý���ȴ���ڿ������ȶ����ڣ�ԭ����(�û�ѧ���P�������˵��)___��
(2)�����������Խ�����ִ��������������й㷺��Ӧ�á���������������价������ȫ���ܵ�Խ��Խ��Ĺ�ע����ԭ����ͼ��ʾ��
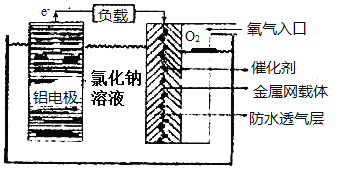
�õ�ص�������Ӧ����ʽΪ___�������缫��������9�ˣ�����һ��ͨ�������������ڱ�״����Ϊ___��
(3)AlCl3��NaN3�ڸ����·�Ӧ���Ƶø��½ṹ�մɵ�����(AlN)��������N2��д����Ӧ��ѧ����ʽΪ___��
(4)��Ԫ�����ڱ��У���λ��������һ���ڣ��뵪Ԫ��ͬ���壬д��AsH3���ӵĵ���ʽΪ___����ͬѹǿ�£�AsH3�ķе�___NH3(��������������С����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������£�����0.1 mol��L��1 NH4HCO3��Һ��pH��7.8����֪����(��̼)�����ķֲ�����(ƽ��ʱ������Ũ��ռ����Ũ��֮�͵ķ���)��pH�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����

A.��pH��9ʱ����Һ�д������й�ϵ��c(NH4��)>c(NH3��H2O)>c(HCO3��)>c(CO32��)
B.��0.2 mol CO2ͨ��1 L 0.3 mol��L��1 NH3��H2O��Һ�г�ַ�Ӧ�����У�c(HCO3��)��3c(H2CO3)��c(CO32��)>0
C.��pH��6.5��������Һ����εμ�����������Һʱ��NH4����HCO3��Ũ����С
D.������֪��������ˮ��ƽ�ⳣ��Kh(HCO3��)��������Ϊ10��7
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���ˮ�������������ֱ������ȼ�ϵ�ء��ش��������⣺
(1)��֪�״��ֽⷴӦ��CH3OH(g)![]() CO(g)��2H2(g) ��H1����90.64 kJ��mol��1��
CO(g)��2H2(g) ��H1����90.64 kJ��mol��1��
ˮ�����任��Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H2����41.20 kJ��mol��1��
CO2(g)��H2(g) ��H2����41.20 kJ��mol��1��
��CH3OH(g)��H2O(g)![]() CO2(g)��3H2(g) ��H3��___________kJ��mol��1��
CO2(g)��3H2(g) ��H3��___________kJ��mol��1��
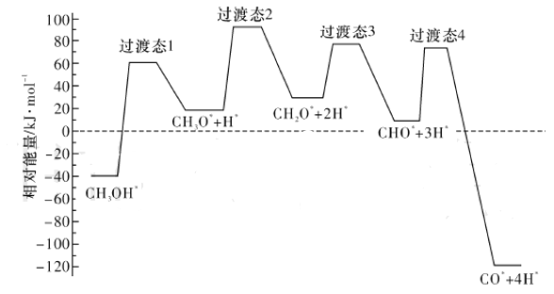
(2)��ѧ��ͨ���ܶȷ��������о��״���ˮ�����������ⷴӦ����ʱ���õ��״���Pd(III)���淢������ʱ�ĸ�·������������Ĺ�ϵ��ͼ��ʾ�����и���Pd(III)�����������*��ע���������л����С�ķ�Ӧ����ʽΪ_____________________________________________��
(3)��0.1MPa�£����ܽ�����Ϊ1 mol��n(CH3OH)��n(H2O)��1��1.3�Ļ���������һ�����ܱ������з�Ӧ��
��ʵ����ˮ�����任��Ӧ���������¶ȵ����������½���ԭ����____________________��
��ƽ��ʱ�����CH3OH�ĺ����ڸ����¶ȷ�Χ�ڼ�С��H2��H2O(g)��CO��CO2������ֵĺ����뷴Ӧ�¶ȵĹ�ϵ��ͼ��ʾ������b��c��Ӧ���ʵĻ�ѧʽ�ֱ�Ϊ________��________��

(4)573.2Kʱ����һ�����ܱ������г���5.00 MPa CH3OHʹ��ֽ⣬t h���ƽ��ʱH2�����ʵ�������Ϊ60%����t h��v(CH3OH)��_____MPa��h��1�����ѹƽ�ⳣ��Kp��_____MPa2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ���¶��£��������ˮϡ��������Һ�ĵ�����������ͼ��ʾ����ش�

��1��д������ĵ��뷽��ʽ___________________________________________��
��2��a��b��c������Һ��������Ũ����С�����˳��Ϊ__________________ ��
��3��a��b��c�����д���ĵ���̶�������_________��
��4��ȡ�ס������ݵ����c�����Һ����������ˮϡ��10������������ˮϡ��100������ϡ�ͺ�ס�������Һ�е�H+Ũ�ȣ�c(H+)��_____ 10c(H+)��������ڡ�����С�ڡ��� �����ڡ���
��5�������백ˮ��Ӧ�����ӷ���ʽ��__________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����FeCl3��Һ��KI��Һ��ϣ�������Ӧ��2Fe3��(aq)��2I��(aq)![]() 2Fe2��(aq)��I2(aq)�����и������ж��������淴Ӧ�ﵽƽ��״̬����
2Fe2��(aq)��I2(aq)�����и������ж��������淴Ӧ�ﵽƽ��״̬����
A. ��Һ��ɫ���ٱ仯
B. c(K��)���ٱ仯
C. c(Fe3��)��c(Fe2��)֮�Ͳ��ٱ仯
D. v��(I��)��2v��(I2)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com