
A��B��C��D��E����5��Ԫ�ء�����գ�
��1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ���Ԫ������Ϊ______��
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ��BԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ______________��C�����ӵĵ����Ų�ʽΪ__________________________��
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ�������D��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ_____________��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ�E��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ________________________��
��1�� ������2�� 1s22s22p63s23p5�� 1s22s22p63s23p6
��3�� Fe��1s22s22p63s23p63d64s2����4�� Cu��1s22s22p63s23p63d104s1
���������������1��AԪ�ػ�̬ԭ�ӵ��������3��δ�ɶԵ��ӣ��������2�����ӣ�����ݹ���ԭ����֪��Ԫ�ص�ԭ��������2��2��3��7����˸�Ԫ������Ϊ����
��2��BԪ�صĸ�һ�����Ӻ�CԪ�ص���һ�����ӵĵ��Ӳ�ṹ�������ͬ������Ar��18��Ԫ�أ�����B����Ԫ�أ�C��KԪ�ء�����ݹ���ԭ����֪��BԪ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p5��C�����ӵĵ����Ų�ʽΪ1s22s22p63s23p6��
��3��DԪ�ص����������ӵ�3d�Dz�Ϊ���������D��ԭ������Ӧ����2��8��8��5��3��26����D��FeԪ�ء����Ը��ݹ���ԭ����֪D��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2��
��4��EԪ�ػ�̬ԭ�ӵ�M��ȫ������N��û�гɶԵ��ӣ�ֻ��һ��δ�ɶԵ��ӣ���EԪ�ص�ԭ��������2��8��18��1��29����E��ͭԪ�ء�����ݹ���ԭ����֪E��Ԫ�ػ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
���㣺����Ԫ���ƶ��Լ���������Ų���д


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E���ֶ�����Ԫ�ص�ԭ��������������Ԫ��A�ĸ�һ�����Ӿ��к�ϡ������Heһ���Ľṹ��Ԫ��B���������������ڲ����������2����Ԫ��C�Ƕ�����Ԫ����ԭ�Ӱ뾶��������Ԫ�أ�Ԫ��D�ڵؿ��еĺ���λ�ڵڶ�λ��Ԫ��E��Ԫ��B���γɾ�����������ṹ�����ʡ���ش��������⣺
��1�������ڱ��У�Ԫ��Bλ�ڵ� ���ڵ� �壻A��C�γɵ����ӻ�����Ļ�ѧʽΪ _________________ �� A��C�γɵ����ӻ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��C�γɵļ����ӵ����ӽṹʾ��ͼΪ ��
��3��D��EԪ���γɵĻ��������ˮ��Ӧ����һ�ֳ�����һ�����壬��д���÷�Ӧ�Ļ�ѧ����ʽ�� ___________ ��
��4������B��ˮ��Ӧ�ǽ�B������õĴ�ʩ֮һ����д���÷�Ӧ�Ļ�ѧ����ʽ�� ______________________________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��14�֣�A��B��C��D��E��F��Ϊ������Ԫ�أ�ԭ���������ε�����AԪ��ԭ�Ӻ��������ӡ�BԪ��ԭ�������������Ǵ�����������2����CԪ���ǵؿ��Ǻ�������Ԫ�ء�D�Ƕ�����Ԫ���н�������ǿ��Ԫ�ء�E��F��λ�����ڣ�F������������ˮ����Ϊ��ǿ���ᡣ
��1���ƶ�B��Ԫ�����ڱ��е�λ�ã� ��
��2��д��A��C�γɵ�10���ӵ������ӻ�ѧʽ�� �����ö�Ӧ�Ļ�ѧ������գ���ͬ��
��3��E��F����Ԫ���зǽ����Խ�ǿ���� ���õ���ʽ��ʾD2C�γɹ���
��4��D��E�γɵ����ε�ˮ��Һ�У������ӵ�Ũ�ȴ�С˳��Ϊ�� ���Ӵ�С���У���
��5����������1molAԪ�صĵ�����CԪ�صĵ��ʻ��ϣ��ų�286kJ��������д����Ӧ���Ȼ�ѧ����ʽ��
��6������A��C��ԭ�Ӹ�����1:1��ɵĻ������֪����Һ��ʹ���Ը��������Һ��ɫ��������0.5mol����Һ�μӵ�100mL 2mol/L���Ը��������Һ�У���Һ��ɫǡ����ȥ���÷�Ӧ�����ӷ���ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ������������������X��Y��Z��W���γɵij����������ڳ����¾�����̬�������ڱ���Z��W�������ڣ�Y������������ˮ���������⻯�ﷴӦ�����Σ���Y�ĺ˵������W��������������ͬ����ش��������⣺
��1��Z�����ӽṹʾ��ͼ�� ��
��2��X��Y��W�����һ�������ԭ�Ӹ���֮��Ϊ4��1��1���仯ѧʽ�к��еĻ�ѧ���� ��
��3��YX3���ӵĿռ乹���� ��Z���⻯����ȶ��Ա�W���⻯����ȶ���_____���ǿ������������
��4��Z2W2�����У�W��Z������8e-�ȶ��ṹ����Z 2W2�ĵ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
W��X��Y��Z�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ����֪W��һ�ֺ��ص�������Ϊ18��������Ϊ10��X��Neԭ�ӵĺ�����������1��Y�ĵ�����һ�ֳ����İ뵼����ϣ�Z�ĵ縺����ͬ��������Ԫ�������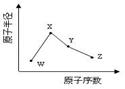
��1��Xλ��Ԫ�����ڱ��е�λ�� ��18W��X�γ�ԭ�Ӹ���1��1�����ʣ���������H2O��Ӧ�Ļ�ѧ����ʽ �����ڻ�ѧʽ�б��������18��
��2��X�ĵ��ʺ�Y�ĵ�����ȣ��۵�ϸߵ���_____(д��ѧʽ)��Z����̬�⻯����廯����ȣ����ȶ�����_______(д��ѧʽ)��
��3��Y��Z�γɵĻ����������ˮ��Ӧ������һ�������һ��ǿ�ᣬ�÷�Ӧ�Ļ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
1886�꣬�¹���������Frieberg����ҵѧԺ��һλ�����ڷ����������������ֵ�һ���¿�ʯ����X��ʾ��XΪ���Ȼ������ʱ����������һ�ֵ�ʱδ֪����Ԫ�أ���Y��ʾ����X��Y�Ļ��ϼ�Ϊ+4������ͨ��ʵ����֤���Լ����ƶϡ���������X�к�����Ԫ�أ����ϼ�Ϊ+1������Ԫ�أ����ϼ�Ϊ-2��������Ag����������Ϊ76��53%�����������м���X���ɵõ�Ag2S��H2S��Y�ĵͼ����Y�Ļ��ϼ�Ϊ+2���������200���100kPa��������ȫת��16��0g��X��Ҫ0��557L�����������������������
��1������Y��Ħ��������ָ��Y��Ԫ�����ڱ��е�λ��
��2��д��X�Ļ�ѧʽ
��3��д��������X��Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D�����ֶ�����Ԫ�أ����ǵ�ԭ������������������AԪ�ص�ԭ��ֻ��һ�����Ӳ㣬��֪A��C��B��D�ֱ�����ͬһ����Ԫ�أ�B��D��Ԫ�ص�ԭ�Ӻ���������֮����A��C��Ԫ��ԭ�Ӻ���������֮�͵Ķ�������֪����Ԫ�صĵ��ʳ��³�ѹ�����������塢���ֹ��塣��ش��������⣺
��1��DԪ����Ԫ�����ڱ��е�λ�ã� ��
��2��BԪ�ر�DԪ�صķǽ�����ǿ�������� ������ţ���
a����Ԫ����ɵĻ�������DԪ��Ϊ���� b�����ʵ��۷е�ĸߵ�
c������������Ӧ��ˮ���������ǿ�� d����̬�⻯����ȶ���
��3����A��B��D����Ԫ���е����ֿɷֱ��γɼס����������ӣ����Ǿ�Ϊ��һ��˫ԭ�Ӻ˵������ӣ��Ҽ����Ӻ���18�����ӣ������Ӻ���10�����ӣ�������ҷ�Ӧ�����ӷ���ʽΪ ��
��4��д��C��D��Ԫ�ص�����������Ӧ��ˮ������ϡ��Һ�ﷴӦ���Ȼ�ѧ����ʽ�� ����֪�˷�Ӧ����1 molH2Oʱ�ų�������Ϊ57.3 kJ����
��5����AԪ�صĵ�����BԪ�صĵ��ʿ����Ƴ�ȼ�ϵ�أ�ȼ�ϵ����װ��KOHŨ��Һ���ö�Ľ������Ե缫����KOH��Һ����M��ͨ��A�ĵ��ʣ�N��ͨ��B�ĵ��ʣ���N���ĵ缫��ӦʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��E���ֶ�����Ԫ�أ����ǵ�ԭ��������������AԪ����ԭ�Ӱ뾶��С��ԭ�ӣ�BԪ�ص�����������Ӧˮ���������⻯������һ����X��D��Aͬ���壬����Eͬ���ڣ�EԪ�ص��������������������������3/4��A��B��D��E������Ԫ�أ�ÿһ����CԪ�ض����γ�Ԫ�ص�ԭ�Ӹ����Ȳ���ͬ�������ֻ������ش��������⣺
��1��C��E��Ԫ����Ƚϣ��ǽ����Խ�ǿ���� (��Ԫ������)��������֤�ý��۵��� (��д���)
A���Ƚ�������Ԫ�ص���̬�⻯��ķе�
B���Ƚ�������Ԫ�ص�ԭ�ӵĵ��Ӳ���
C���Ƚ�������Ԫ�ص���̬�⻯����ȶ���
D���Ƚ�������Ԫ�صĵ������⻯�ϵ�����
��2��C��D��Ԫ���γɵ�ԭ�Ӹ�����Ϊ1:1�Ļ�������C��E��Ԫ���γɵ����ֻ����ﶼ�ܷ�Ӧ������һ�ֹ�ͬ�IJ��д����������Ӧ�Ļ�ѧ����ʽ�� �� ��
��3��ij��ҵ�����ĺ��ķ�Ӧ�� ��2EC2(g) �� C2(g)  2EC3(g) ��H��0���ش��������⣺
2EC3(g) ��H��0���ش��������⣺
�ٴ˷�Ӧ��ƽ�ⳣ������ʽΪK= �������¶ȵ����ߣ�����ƽ�ⳣ��
(���������С�����䡱)��
�ڽ�һ������EC2(g)��C2(g)����1L�ܱ������У���һ�������´ﵽƽ�⣬���EC2Ϊ0.11mol��C2Ϊ0.05mol��EC3Ϊ0.12mol������������£���Ӧ��ƽ�ⳣ��K= ��EC2��ת��ΪEC3ת����= ��
��4��A��C��E����γɼס��������������Ǿ�Ϊ��һ��˫ԭ�������ӣ��Ҽ���18�����ӣ�����10�����ӣ�������ҷ�Ӧ�����ӷ���ʽΪ ��
��5�����ڻ���ƽ�����װ��Һ̬B2A4��Һ̬A2C2����֪0.4mol.Һ̬B2A4������Һ̬A2C2��Ӧ��������̬B2����̬A2C���ų�256.6kJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��B2A4�ֳ�Ϊ�£���һ�ֿ�ȼ��Һ�壬��һ����ȼ�ϵ����һ�ּ���ȼ�ϵ�أ��������Һ��20%��30%��KOH��Һ���õ�طŵ�ʱ�ĵ缫��ӦʽΪ������ ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��ԭ��������������Ķ���������Ԫ�أ�A��C��Ԫ�����ڱ��е����λ����ͼ��AԪ��������������ϵĵ�����֮��Ϊ3��BΪ�ؿ��к������Ľ���Ԫ�ء�
| A | |
| | C |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com