
ЁОЬтФПЁПЕЊЪЧЕиЧђЩЯКЌСПЗсИЛЕФвЛжждЊЫиЃЌЕЊМАЦфЛЏКЯЮядкЙЄХЉвЕЩњВњЁЂЩњЛюжагазХживЊзїгУЁЃ
ЃЈ1ЃЉЩЯЭМЪЧ![]() КЭ
КЭ![]() ЗДгІЩњГЩ
ЗДгІЩњГЩ![]() Й§ГЬжаФмСПЕФБфЛЏЪОвтЭМЃЌЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧ_______ЁЃ
Й§ГЬжаФмСПЕФБфЛЏЪОвтЭМЃЌЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧ_______ЁЃ
a. ЗДгІЮяЕФзмФмСПБШЩњГЩЮяЕФзмФмСПИп
b. ЗДгІЮяЕФЛюЛЏФмБШЩњГЩЮяЕФЛюЛЏФмИп
c. ЗДгІЮяЕФзмМќФмБШЩњГЩЮяЕФзмМќФмИп
d. ИУЗДгІЮЊьидіЗДгІ
ЃЈ2ЃЉЧыаДГі![]() КЭ
КЭ![]() ЗДгІЕФШШЛЏбЇЗНГЬЪНЃК_______ЃЌОіЖЈИУЗДгІНјааЗНЯђЕФжївЊХаОнЮЊ________ЁЃ
ЗДгІЕФШШЛЏбЇЗНГЬЪНЃК_______ЃЌОіЖЈИУЗДгІНјааЗНЯђЕФжївЊХаОнЮЊ________ЁЃ
ЃЈ3ЃЉЪдИљОнБэжаМАЭМжаЪ§ОнМЦЫу![]() ЕФМќФм______________ kJ/molЃЛ
ЕФМќФм______________ kJ/molЃЛ
ЛЏбЇМќ |
|
|
МќФм/ kJ/mol | 390 | 943 |
ЃЈ4ЃЉгУ![]() ДпЛЏЛЙд
ДпЛЏЛЙд![]() ЛЙПЩвдЯћГ§ЕЊбѕЛЏЮяЕФЮлШОЁЃвбжЊЃК
ЛЙПЩвдЯћГ§ЕЊбѕЛЏЮяЕФЮлШОЁЃвбжЊЃК
4NH3(g)+3O2(g)=2N2(g)+6H2O(g)ЃЛЁїH1=-akJ/mol
N2(g)+O2(g)=2NO(g)ЃЛЁїH2=-bkJ/mol
Шє1molNH3ЛЙдNOжСN2ЃЌдђИУЗДгІЙ§ГЬжаЕФЗДгІШШЁїH3=_____________kJ/mol(гУКЌaЁЂbЕФЪНзгБэЪО)ЁЃ
ЁОД№АИЁПa ![]() ЃЈКЯРэМДПЩЃЉ ьЪХаОнЛђІЄH<0 435
ЃЈКЯРэМДПЩЃЉ ьЪХаОнЛђІЄH<0 435 ![]()
ЁОНтЮіЁП
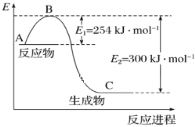
(1)ИљОнЭМЯѓЃЌИУЗДгІЮяЕФзмФмСПБШЩњГЩЮяЕФзмФмСПИпЃЌНсКЯN2(g)+3H2(g)2NH3(g)ЗжЮіХаЖЯЃЛ
(2)ЯШЧѓГіДЫЗДгІЕФьЪБфЃЌИљОнШШЛЏбЇЗНГЬЪНЕФЪщаДЙцдђдйаДГіШШЛЏбЇЗНГЬЪНЃЛИУЗДгІКѓЦјЬхЕФЮяжЪЕФСПМѕЩйЃЌНсКЯИДКЯХаОнЗжЮіНтД№ЃЛ
(3)ИљОнЗДгІШШЕШгкЗДгІЮяЕФзмМќФмМѕШЅЩњГЩЮяЕФзмМќФмМЦЫуЃЛ
(4)РћгУИЧЫЙЖЈТЩЗжЮіМЦЫуЁЃ
(1)ИљОнЭМЯѓЃЌЗДгІЮяЕФзмФмСПБШЩњГЩЮяЕФзмФмСПИпЃЌЫЕУїИУЗДгІЮЊЗХШШЗДгІЃЌЗДгІЮяЕФзмМќФмаЁгкЩњГЩЮяЕФзмМќФмЃЛЗДгІЮяЕФЛюЛЏФмЮЊ254 kJ/molЃЌЩњГЩЮяЕФЛюЛЏФмЮЊ300 kJ/molЃЌЗДгІЮяЕФЛюЛЏФмБШЩњГЩЮяЕФЛюЛЏФмЕЭЃЌЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊN2(g)+3H2(g)2NH3(g)ЃЌе§ЗДгІЪЧвЛИіьиМѕаЁЕФЗДгІЃЌе§ШЗЕФжЛгаaЃЌЙЪД№АИЮЊЃКaЃЛ
(2)ЗДгІЮязмФмСПДѓгкЩњГЩЮязмФмСПЃЌгІЮЊЗХШШЗДгІЃЌЩњГЩ1molАБЦјЗХГі46kJШШСПЃЌдђЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊN2(g)+3H2(g)2NH3(g)ЁїH=-92kJ/molЃЌИУЗДгІЪЧвЛИіьиМѕаЁЕФЗДгІЃЌОіЖЈИУЗДгІНјааЗНЯђЕФжївЊХаОнЮЊьЪХаОнЃЌЙЪД№АИЮЊЃКN2(g)+3H2(g)2NH3(g)ЁїH=-92kJ/molЃЛьЪХаОнЃЛ
(3)ЗДгІШШЕШгкЗДгІЮяЕФзмМќФмМѕШЅЩњГЩЮяЕФзмМќФмЃЌЩшH-HЕФМќФмЮЊxЃЌдђ943 kJ/mol +3 x-6ЁС390 kJ/mol =-92 kJ/molЃЌx=435 kJ/molЃЌЙЪД№АИЮЊЃК435ЃЛ
(4)Ђй4NH3(g)+3O2(g)=2N2(g)+6H2O(g)ЃЛЁїH1=-akJ/molЃЌЂкN2(g)+O2(g)=2NO(g)ЃЛЁїH2=-bkJ/molЃЌИљОнИЧЫЙЖЈТЩЃЌНЋ![]() ПЩЕУЃКNH3(g)+
ПЩЕУЃКNH3(g)+![]() NO(g)=
NO(g)=![]() N2(g)+
N2(g)+![]() H2O(g)ЁїH3=
H2O(g)ЁїH3=![]() kJ/molЃЌЙЪД№АИЮЊЃК
kJ/molЃЌЙЪД№АИЮЊЃК![]() ЁЃ
ЁЃ


 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПИљОнвЊЧѓЭъГЩЯТСаУПвЛаЁЬт
ЃЈ1ЃЉИљОнЯЕЭГУќУћЗЈЃЌ![]() ЕФУћГЦЪЧ___ЃЛ
ЕФУћГЦЪЧ___ЃЛ
ЃЈ2ЃЉИљОнЯЕЭГУќУћЗЈ![]() ЕФУћГЦЪЧ___ЃЛ
ЕФУћГЦЪЧ___ЃЛ
ЃЈ3ЃЉИљОнЯЕЭГУќУћЗЈ ЕФУћГЦЪЧ___ЃЛ
ЕФУћГЦЪЧ___ЃЛ
ЃЈ4ЃЉ КЫДХЙВеёЧтЦзЮќЪеЗхУцЛ§жЎБШЪЧ___ЁЃ
КЫДХЙВеёЧтЦзЮќЪеЗхУцЛ§жЎБШЪЧ___ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђЕШЮяжЪЕФСПХЈЖШЕФHClЁЂ![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ![]() ЛьКЯШмвКжаж№ЕЮМгШы
ЛьКЯШмвКжаж№ЕЮМгШы![]() ЕФNaOHШмвКЃЌЩњГЩГСЕэЕФЮяжЪЕФСПгыМгШыNaOHШмвКЕФЬхЛ§ЙиЯЕШчЭМЫљЪОЁЃЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧ
ЕФNaOHШмвКЃЌЩњГЩГСЕэЕФЮяжЪЕФСПгыМгШыNaOHШмвКЕФЬхЛ§ЙиЯЕШчЭМЫљЪОЁЃЯТСагаЙиЫЕЗЈе§ШЗЕФЪЧ ![]()
![]()
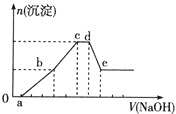
A.дк![]() ЖЮМгШыЕФNaOHШмвКгы
ЖЮМгШыЕФNaOHШмвКгы![]() ЗДгІ
ЗДгІ
B.дк![]() ЖЮМгШыNaOHШмвКЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ
ЖЮМгШыNaOHШмвКЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ![]()
C.дк![]() ЖЮМгШыNaOHШмвКЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ
ЖЮМгШыNaOHШмвКЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ![]()
D.дкЕЮМгNaOHШмвКШЋЙ§ГЬжажївЊСЃзгВЮгыЗДгІЕФЯШКѓЫГађЪЧ![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ![]() ЁЂ
ЁЂ![]()
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПNa2FeO4ЁЂLi4YiO12КЭLiFePO4ОљПЩгУзїЕчМЋВФСЯЁЃЧыЛиД№ЯТСаЮЪЬтЃК
ЂХЛљЬЌFe3+ЕФМлВуЕчзгЙьЕРБэДяЪНЮЊ________ЃЛЭЌжмЦкдЊЫижаЃЌЛљЬЌдзгЕФЮДГЩЖдЕчзгЪ§гыЛљЬЌFe3+ЯрЭЌЕФдЊЫиЮЊ________ЁЃ
ЂЦPO43ЃЕФПеМфЙЙаЭЮЊ________ЃЌЦфжаPдзгЕФдгЛЏЗНЪНЮЊ________ЃЛаДГівЛжжгыPO43ЃЛЅЮЊЕШЕчзгЬхЧвжааФдзггыPВЛЭЌжїзхЕФвѕРызгЃК________(ЬюРызгЗћКХ)ЁЃ
ЂЧ[Ti(H2O)6]Cl3ЮЊзЯЩЋОЇЬхЃЌЦфжа1molбєРызгжаЫљКЌІФМќЕФЪ§ФПЮЊ________ЃЛХфЮЛМќжаЬсЙЉЙТЕчзгЖдЕФдзгЮЊ________(ЬюдЊЫиЗћКХ)ЁЃ
ЂШвбжЊЮяжЪМфЕФзЊЛЏЙиЯЕШчЭММзЫљЪОЃЌЦфжаaЁЂcОљДѓгк0ЁЃ
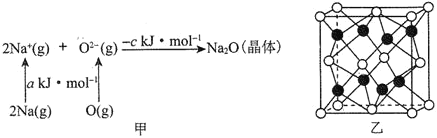
ЂйЛљЬЌNaдзгЕФЕквЛЕчРыФмПЩБэЪОЮЊ________ЁЃ
ЂкЯрЭЌЬѕМўЯТЃЌLi2OЕФОЇИёФм________(ЬюЁА>ЁБЁА<ЁБЛђЁА=ЁБ)ckJmol-1ЃЌдвђЮЊ________________________________ЁЃ
ЂлNa2OЕФСЂЗНОЇАћНсЙЙШчЭМввЫљЪОЁЃШєНєСкЕФСНИіNa+жЎМфЕФОрРыЮЊd pmЃЌАЂЗќМгЕТТоГЃЪ§ЕФжЕЮЊNAЃЌОЇЬхЕФУмЖШЮЊІб gcm-3ЃЌдђNaЕФФІЖћжЪСППЩБэЪОЮЊ_______gmol-1(гУКЌгаdЁЂІбЁЂNAЕФДњЪ§ЪНБэЪО)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊAЁЂBЁЂCЁЂDЫФжжЖЬжмЦкдЊЫиЃЌЫќУЧЕФКЫЕчКЩЪ§вРДЮдіДѓЃЌAгыCдзгЕФЛљЬЌЕчзгХХВМжаLФмВуЖМгаСНИіЮДГЩЖдЕчзгЃЌCЁЂDЭЌжїзхЃЌEЁЂFЖМЪЧЕкЫФжмЦкдЊЫиЃЌEдзгЕФЛљЬЌЕчзгХХВМжага4ИіЮДГЩЖдЕчзгЃЌFдзгГ§зюЭтФмВужЛга1ИіЕчзгЭтЃЌЦфгрИїФмВуОљЮЊШЋГфТњЁЃИљОнвдЩЯаХЯЂЬюПеЃК
ЃЈ1ЃЉЛљЬЌDдзгжаЃЌЕчзгеМОнЕФзюИпФмВуЗћКХ ______ ЃЌИУФмВуОпгаЕФдзгЙьЕРЪ§ЮЊ ______ЁЃ
ЃЈ2ЃЉE2+РызгЕФМлВуЕчзгХХВМЭМЪЧ ______ ЃЌFдзгЕФЕчзгХХВМЪНЪЧ ______ ЁЃ
ЃЈ3ЃЉAдЊЫиЕФзюИпМлбѕЛЏЮяЖдгІЕФЫЎЛЏЮяжааФдзгВЩШЁЕФЙьЕРдгЛЏЗНЪНЮЊ ______ ЃЌBдЊЫиЕФЦјЬЌЧтЛЏЮяЕФVSEPRФЃаЭЮЊ ______ ЁЃ
ЃЈ4ЃЉЛЏКЯЮяAC2ЁЂB2CКЭвѕРызгDAB-ЛЅЮЊЕШЕчзгЬхЃЌЫќУЧНсЙЙЯрЫЦЃЌDAB-ЕФЕчзгЪНЮЊ ______ ЁЃ
ЃЈ5ЃЉХфКЯЮяМзЕФбцЩЋЗДгІГЪзЯЩЋЃЌЦфФкНчгЩжааФРызгE3+гыХфЮЛЬхAB-ЙЙГЩЃЌХфЮЛЪ§ЮЊ6ЃЌМзЕФЫЎШмвКПЩвдгУгкЪЕбщЪвжаE2+РызгЕФЖЈадМьбщЃЌМьбщE2+РызгЕФРызгЗНГЬЪНЮЊ ______ ЁЃ
ЃЈ6ЃЉФГжжЛЏКЯЮягЩDЃЌEЃЌFШ§жждЊЫизщГЩЃЌЦфОЇАћШчЭМЫљЪОЃЌдђЦфЛЏбЇЪНЮЊ ______ЃЌИУОЇАћЩЯЯТЕзУцЮЊе§ЗНаЮЃЌВрУцгыЕзУцДЙжБЃЌИљОнЭМжаЫљЪОЕФЪ§ОнСаЪНМЦЫуИУОЇЬхЕФУмЖШd= ______ g/cm3(СаГіМЦЫуЪНЃЌЮоашМЦЫу)ЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬЋбєФмЕчГиАхВФСЯГ§ЕЅОЇЙшЭтЃЌЛЙгаЕЊЁЂХ№ЁЂЮјЁЂюбЁЂюмЁЂИЦЕШдЊЫизщГЩЕФЛЏбЇЮяжЪЁЃ
ЂХИЦдзгЛљЬЌЪБЕФЕчзгХХВМЪНЮЊ____________________ЃЌН№ЪєюмЖбЛ§ЗНЪНгыУОЯрЫЦЃЌЖМЪєгкСљЗНзюУмЖбЛ§ЃЌЦфХфЮЛЪ§ЪЧ____ЁЃ
ЂЦЕЊдЊЫиЕФЕквЛЕчРыФмдкЭЌжмЦкжа(ЯЁгаЦјЬхГ§Эт)ДгДѓЕНаЁХХЕк___ЮЛЃЛаДГігыNO3ЃЛЅЮЊЕШЕчзгЬхЕФвЛжжЗЧМЋадЗжзгЕФЛЏбЇЪН__________ЁЃ
ЂЧОЇЬхХ№ЕФНсЙЙЕЅдЊЪЧе§ЖўЪЎУцЬхЃЌУПИіЕЅдЊжага12ИіХ№дзг(ШчЭМ)ЃЌЦфжагаСНИідзгЮЊ10BЃЌЦфгрЮЊ11BЃЌдђИУНсЙЙЕЅдЊга_____________жжВЛЭЌЕФНсЙЙРраЭЁЃМКжЊХ№Ыс(H3BO3)ЮЊвЛдЊШѕЫсЃЌНтЪЭЦфЮЊвЛдЊШѕЫсЕФдвђ______________ЁЃХ№ЫсЕФНсЙЙгыЪЏФЋЯрЫЦЃЌВуФкЕФЗжзгвдЧтМќЯрСЌЃЌКЌ1 molХ№ЫсЕФОЇЬхжага___molЧтМќЁЃ

ЂШЮјЪЧЖЏЮяЬхБиашЕФгЊбјдЊЫиЁЃSeO2ЪЧЮјЕФживЊЛЏКЯЮяЃЌSeO2ЕФМлВуЕчзгЖдЛЅГтФЃаЭЪЧ_______________ЁЃ
ЂЩдкХЈЕФTiCl3ЕФбЮЫсШмвКжаМгШыввУбЃЌВЂЭЈШыHC1жСБЅКЭЃЌПЩЕУЕНХфЮЛЪ§ЮЊ6ЃЌзщГЩЮЊ TiCl36H2OЕФОЇЬхЃЌИУОЇЬхжаСНжжХфЬхЕФЮяжЪЕФСПжЎБШЮЊ1ЃК5ЃЌдђИУХфРызгЕФЛЏбЇЪНЮЊЃК__________________ЁЃ
ЂЪюмОЇЬхЕФвЛжжОЇАћЪЧвЛжжЬхаФСЂЗННсЙЙ(ШчЭМЫљЪО)ЃЌШєИУОЇАћЕФБпГЄЮЊa nmЃЌУмЖШЮЊІб gcm-3ЃЌNAБэЪОАЂЗќМгЕТТоГЃЪ§ЕФжЕЃЌдђюмЕФЯрЖддзгжЪСППЩБэЪОЮЊ_________________ЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФГЮТЖШЯТЃЌ![]() КЭ
КЭ![]() ЕФЕчРыГЃЪ§ЗжБ№ЮЊ
ЕФЕчРыГЃЪ§ЗжБ№ЮЊ![]() КЭ
КЭ![]() ЁЃНЋ
ЁЃНЋ![]() КЭЬхЛ§ОљЯрЭЌЕФСНжжЫсШмвКЗжБ№ЯЁЪЭЃЌЦф
КЭЬхЛ§ОљЯрЭЌЕФСНжжЫсШмвКЗжБ№ЯЁЪЭЃЌЦф![]() ЫцМгЫЎЬхЛ§ЕФБфЛЏШчЭМЫљЪОЁЃЯТСаа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ
ЫцМгЫЎЬхЛ§ЕФБфЛЏШчЭМЫљЪОЁЃЯТСаа№Ъіе§ШЗЕФЪЧЃЈ ЃЉ
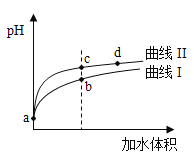
A. ЧњЯпЂёДњБэ![]() ШмвК
ШмвК
B. ШмвКжаЫЎЕФЕчРыГЬЖШЃКbЕуЃОcЕу
C. ДгcЕуЕНdЕуЃЌШмвКжа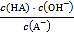 БЃГжВЛБфЃЈЦфжа
БЃГжВЛБфЃЈЦфжа![]() ЁЂ
ЁЂ![]() ЗжБ№ДњБэЯргІЕФЫсКЭЫсИљРызгЃЉ
ЗжБ№ДњБэЯргІЕФЫсКЭЫсИљРызгЃЉ
D. ЯрЭЌЬхЛ§aЕуЕФСНШмвКЗжБ№гы![]() ЧЁКУжаКЭКѓЃЌШмвКжа
ЧЁКУжаКЭКѓЃЌШмвКжа![]() ЯрЭЌ
ЯрЭЌ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСазАжУжагаЛњЮябљЦЗдкЕчТЏжаГфЗжШМЩеЃЌЭЈЙ§ВтЖЈЩњГЩЕФCO2КЭH2OЕФжЪСПРДШЗЖЈгаЛњЮязщГЩЁЃ

ЧыЛиД№ЯТСаЮЪЬтЃК
(1)AзАжУЪЧЬсЙЉЪЕбщЫљашЕФO2ЃЌаДГігаЙиЗДгІЕФЛЏбЇЗНГЬЪН__________ЃЛBзАжУжаЪдМСXПЩбЁгУ_________ЁЃ
(2)ШєзМШЗГЦШЁ0.44gбљЦЗ(жЛКЌCЁЂHЁЂOШ§жждЊЫижаЕФСНжжЛђШ§жж)ЃЌГфЗжЗДгІКѓЃЌDЙмжЪСПдіМг0.36gЃЌEЙмжЪСПдіМг0.88gЃЌдђИУгаЛњЮяЕФЪЕбщЪНЮЊ________ЁЃ
(3)вЊШЗЖЈИУгаЛњЮяЕФЗжзгЪНЃЌЛЙашжЊЕРИУгаЛњЮяЕФ_____________ЃЌОВтЖЈЦфеєЦјУмЖШЪЧЯрЭЌЬѕМўЯТH2ЕФ22БЖЃЌдђЦфЗжзгЪНЮЊ___________ЁЃ
(4)ШєИУгаЛњЮяЕФКЫДХЙВеёЧтЦзШчЭМЫљЪОЃЌдђЦфНсЙЙМђЪНЮЊ_____________ЃЛ
ШєИУгаЛњЮяжЛгавЛжжЛЏбЇЛЗОГЕФЧтдзгЃЌдђЦфНсЙЙМђЪНЮЊ___________ЁЃ
(5)ФГЭЌбЇШЯЮЊEКЭПеЦјЯрЭЈЃЌЛсгАЯьВтЖЈНсЙћзМШЗадЃЌгІдкEКѓдйдіМгвЛИіEзАжУЃЌЦфжївЊФПЕФЪЧ______ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПГЃЮТЯТЃЌгУ0.10 molЁЄLЃ1 KOHШмвКЕЮЖЈ10.00 mL 0.10 molЁЄL-1 H2C2O4(ЖўдЊШѕЫс)ШмвКЫљЕУЕЮЖЈЧњЯпШчЭМ(ЛьКЯШмвКЕФЬхЛ§ПЩПДГЩСНепШмвКЕФЬхЛ§жЎКЭ)ЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
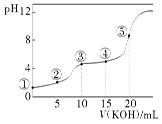
A.ЕуЂкЫљЪОШмвКжаЃКc(KЃЋ)ЃЋc(HЃЋ)ЃНc(HC2O4-)ЃЋc(C2O42-)ЃЋc(OHЃ)
B.ЕуЂлЫљЪОШмвКжаЃКc(KЃЋ)ЃОc(HC2O4-)ЃОcЃЈH2C2O4ЃЉЃОc(C2O42-)
C.ЕуЂмЫљЪОШмвКжаЃКc(KЃЋ)ЃЋc(H2C2O4)ЃЋc(HC2O4-)ЃЋc(C2O42-)ЃН0.10 molЁЄL-1
D.ЕуЂнЫљЪОШмвКжаЃКc(OHЃ)= c(HЃЋ)+ c(HC2O4-)+ c(C2O42-)
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com