
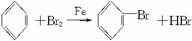
�����Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�
(1)д��A�з�Ӧ�Ļ�ѧ����ʽ___________________________________________��
(2)�۲쵽A�е�������_______________________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����__________________��д���йصĻ�ѧ����ʽ_______________________________________��
(4)C��ʢ��CCl4��������____________________________________________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���______________��������__________________________________________________________________��
(1)C6H6+ Br2 ![]() C6H5Br + HBr��
C6H5Br + HBr��
(2)��ӦҺ�� �к���ɫ�������A����
(3)��ȥ�����屽�е���
Br2+2NaOH![]() NaBr + NaBrO + H2O
NaBr + NaBrO + H2O
��2Br2+ 6NaOH![]() 5NaBr + NaBrO3+ 3H2O
5NaBr + NaBrO3+ 3H2O
(4)��ȥ�廯�������е�������
(5)ʯ����Һ����Һ���ɫ
������������Ҫ�����屽�Ʊ������е�ϸ�����⡣���ڱ���Һ��ķе�ϵͣ��ҷ�Ӧ![]() ��Ϊ���ȷ�Ӧ����A�й۲쵽������Ϊ����ӦҺ�У��к���ɫ�������A������HBr�л��е�Br2�ھ���CCl4 ʱ�����գ����Ʊ����屽�г�����Br2��һ�����NaOH��Һ�У�������Ӧ
��Ϊ���ȷ�Ӧ����A�й۲쵽������Ϊ����ӦҺ�У��к���ɫ�������A������HBr�л��е�Br2�ھ���CCl4 ʱ�����գ����Ʊ����屽�г�����Br2��һ�����NaOH��Һ�У�������Ӧ![]() ����ȥ��
����ȥ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����Һ©���м��뱽��Һ�壬�ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�

(1)д��A�з�Ӧ�Ļ�ѧ����ʽ________________________________________________��
(2)�۲쵽A�е�������______________________________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����______________��д���йصĻ�ѧ����ʽ_________________________________________________________��
(4)C��ʢ��CCl4��������_____________________________________________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м��룬������__________
________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)д��A�з�Ӧ�Ļ�ѧ����ʽ_________________________________��
(2)�۲쵽A�е�������______________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����___________________��д���йصĻ�ѧ����ʽ________________��
(4)C��ʢ��CCl4��������______________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���_____________��������______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)д��A�з�Ӧ�Ļ�ѧ����ʽ______________________________________________��
(2)�۲쵽A�е�������__________________________________________________��
(3)ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ����_______________��д���йصĻ�ѧ����ʽ_____________________________________________________��
(4)C��ʢ��CCl4��������_______________________________________________��
(5)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤������һ����֤�ķ��������Թ�D�м���___________________________��������_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣�ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�� ���ٽ����Һ�������뷴Ӧ��A(A�¶˻����ر�)�С�
(1) д��A�з�Ӧ�Ļ�ѧ����ʽ______________________
(2) ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������
Ŀ����______ ___�� д���йصĻ�ѧ����ʽ��_______________ ____
(3) C��ʢ��CCl4�������� ��
(4)��֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ������
�Թ� D�м���AgNO3��Һ������������ɫ����������֤������һ����
֤�ķ��������Թ�D�м���______ ___�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ���˰�У�߶���ѧ�������������ƻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧ����С������ͼװ����ȡ�屽�������Һ©���м��뱽��Һ�� ���ٽ����Һ�������뷴Ӧ��A��A�¶˻����رգ��С�
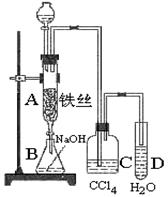
��1��д��A�з�Ӧ�Ļ�ѧ����ʽ
��ʵ�����ʱ����A�¶˵Ļ������÷�ӦҺ����B�У������Ŀ���� ��д���йط�Ӧ�Ļ�ѧ����ʽ ��
�� C��ʢ��CCl4�������� ��
��4����֤������Һ�巢������ȡ����Ӧ�������Ǽӳɷ�Ӧ�������Թ�D�м���AgNO3��Һ������������ɫ����������֤���÷�ӦΪȡ����Ӧ����һ����֤�ķ��������Թ�D�м��� �������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com