
ij�����ų��ķ�ˮ�У����ⶨ��0.012mol��L��1���������8��10��4mol��L��1��H�������ü��������Ƶķ�����ȥ�壬��ѧ����ʽΪ��Na2SO3��Br2��H2O === Na2SO4��2HBr�������������ַ�ˮ5L���ʣ�
(1)�����0.05mol��L��1��Na2SO3��Һ���������ܰ��������
(2)�������ˮ��H�������ʵ���Ũ���Ƕ��٣�(�ٶ���Һ�����Ϊ����֮��)��
��1��Na2SO3��Br2��H2O===Na2SO4��2HBr
1 1
0.05��V 0.012��5
V��1.2(L)������������������������������������������������������2�֣�
��2��Na2SO3��Br2��H2O===Na2SO4��2HBr
1 2
0.05��1.2 x
X��1.2(mol)��������������������������������������������������������������2�֣�
��Ӧ��n(H��) ��0.0008��5 + 0.12��0.124��mol��
V��5 + 1.2��6.2��L��������������������������������������������������2�֣�
![]() ��������
��������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ����ѧ����ñ�����ѧ�˽̰� �˽̰� ���ͣ�038
ij�����ų��ķ�ˮ�к�Cl2��Ϊ��ȥ��ˮ�е������ȣ���ʹ��ˮ������ԣ�������ͼ��ʾ�ķ���������ˮ����A��B���ֱ�ע��һ�������ķ��ռ���Һ������������Һ������
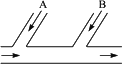
(1)��д����������Ӧ�Ļ�ѧ����ʽ��ָ�������ڷ�Ӧ�е������Ƿ���ͬ��
(2)��д��A��B��Ӧ���봦��Һ�Ļ�ѧʽ�ֱ���________��________����˵��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�ڷ�ˮ�ų��ܵ�A��B���ֱ�ע��һ�������ķ��ռ���Һ������������Һ��
�����
��1��A��B��Ӧ��������ʵĻ�ѧʽ��A_________��B_________��
��2��A��B������Ӧ�����ӷ���ʽ��
A��________________________________________________________________��
B��________________________________________________________________��
��3��ÿ����1 m3�ĸ÷�ˮ�������0.05 mol��L-1��Na2SO3��Һ������Ƕ��٣�
��4����ȥ�Ⱥ�ķ�ˮ��H+��Ũ��ԼΪ���٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

(1)��д����������Ӧ�Ļ�ѧ����ʽ��ָ�������ڷ�Ӧ�е������Ƿ���ͬ��_______________________________________________________________
(2)��д��A��B��Ӧ���봦��Һ�Ļ�ѧʽ�ֱ���________________��________________����˵��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ĩ�� ���ͣ�������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com