
ij����С��������ͼװ�ý����Ҵ��Ĵ�����ʵ�飬ͼ������̨��װ������ȥ���ֺ��߱�ʾ�齺�ܣ�����д���пհף�
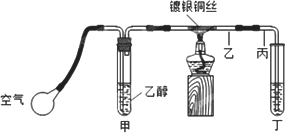
(1)��װ�ó�������70��80���ˮԡ�У�Ŀ����________��
(2)ʵ��ʱ���ȼ��Ȳ��������еĶ���ͭ˿��Լ1���Ӻ�����������ʱͭ˿���ʺ���״̬��Ȼ��Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������
�÷�Ӧ�Ļ�ѧ����ʽΪ________��
(3)Ϊ�˿��ƺù����ٶȣ�Ӧ�۲��װ����________��
(4)ʵ���Ҽ��鶡����������Լ���________����Ӧʵ��������________��
 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012��ɽ���ij�ݷ��ʵ����и߶�������ģ����Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��ÿ��2�֣���18�֣�
��һ��ʵ��������ͼװ����ȡ�����屽������д���пհס�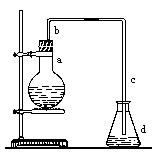
��1������ƿa��װ���Լ��� ��
��2����ֱ����b�����ã� ��
��3�������������c���¿ڿɷ��û��Һ���У� ����ɡ��� ����
��4����Ӧ��Ϻ�����ƿd�еμ�AgNO3��Һ���йط�Ӧ�����ӷ���ʽ�� ��
������ij����С��������ͼװ�ý����Ҵ��Ĵ�����ʵ�鲢��ȡ��ȩ��ͼ������̨��װ������ȥ���ֺ��߱�ʾ�齺�ܡ�����д���пհף�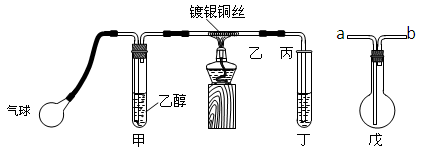
��1����װ�ó�������70��80���ˮԡ�У�Ŀ����
��2��ʵ��ʱ���ȼ��Ȳ��������еĶ���ͭ˿��Լ1���Ӻ�����������ʱͭ˿���ʺ���״̬�����Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������
�Ҵ��Ĵ�������Ӧ��________��Ӧ������ȡ������ȡ�����
�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ�����
������װ���е��ܴ��ţ����ҽ� ��_______�ӱ���
�����ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թܶ����� _____ �С�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�����п�����и�һ��ѧ��ѧ�ڳ�����ѧ�Ծ����������� ���ͣ������
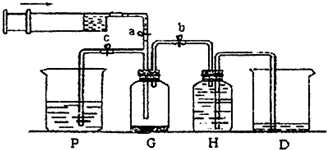
ij����С��������ͼ��ʾװ����ȡ�������ṩ���Լ��У�Ũ���ᡢ����ʳ��ˮ������������Һ��������ع��塣��Ӧ�Ļ�ѧ����ʽΪ��
2KMnO4��+ 16HCl��Ũ���� 2KCl + 2MnCl2��+ 5Cl2��+ 8H2O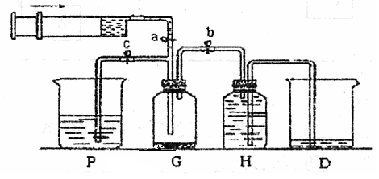
�Իش��������⣺
��1���ڷ�Ӧ2KMnO4 + 16HCl��Ũ���� 2KCl + 2MnCl2 + 5Cl2��+ 8H2O�У�HCl���ֵ�����Ϊ________��_________.�����ɵ�Cl2�����5.6L����״���£�,��ת�Ƶĵ��ӵĸ���________(����٤��������NA����ʾ)
��2��װ��H��ʢ�ŵ��Լ��� ��װ��P��ʢ�ŵ��Լ��� ��
��3��β������ʱ�رյ��ɼ�a�͵��ɼ� �����ɼ� ��
��4������β��ʱ��������Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��������ѵ���и߶���ѧ�ڵ����ο��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ÿ��2�֣���18�֣�
��һ��ʵ��������ͼװ����ȡ�����屽������д���пհס�
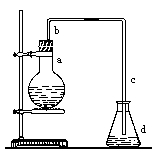
��1������ƿa��װ���Լ��� ��
��2����ֱ����b�����ã� ��
��3�������������c���¿ڿɷ��û��Һ���У� ����ɡ��� ������
��4����Ӧ��Ϻ�����ƿd�еμ�AgNO3��Һ���йط�Ӧ�����ӷ���ʽ�� ��
������ij����С��������ͼװ�ý����Ҵ��Ĵ�����ʵ�鲢��ȡ��ȩ��ͼ������̨��װ������ȥ���ֺ��߱�ʾ�齺�ܡ�����д���пհף�
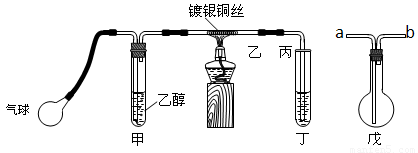
��1����װ�ó�������70��80���ˮԡ�У�Ŀ���� ��
��2��ʵ��ʱ���ȼ��Ȳ��������еĶ���ͭ˿��Լ1���Ӻ�����������ʱͭ˿���ʺ���״̬�����Ѿƾ��Ƴ��ߣ�����һ���Ĺ����ٶȣ�ͭ˿�ܳ�ʱ�䱣�ֺ���ֱ��ʵ�������
�Ҵ��Ĵ�������Ӧ��________��Ӧ������ȡ������ȡ�����
�÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3�����Թܶ�����ˮ���ղ����Ҫ�ڵ����ҡ���֮�������װ�ã������ӷ�����
������װ���е��ܴ��ţ����ҽ� ��_______�ӱ���
�����ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թܶ����� _____ �С�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com