
���з�Ӧ�У�һ�������Է����е���
A.2KClO3(s)= 2KCl(s)+ 3O2(g) ��H = ��78.03 kJ��mol��1����S = 494.4 J��mol��1��K��1
B.CO(g)=
C(s,ʯī)+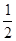 O2(g)
��H = 110.5
kJ��mol��1����S = ��89.4 J��mol��1��K��1
O2(g)
��H = 110.5
kJ��mol��1����S = ��89.4 J��mol��1��K��1
C.4Fe(OH)2(s)+2H2O(l)+O2(g)= 4Fe(OH)3(s)
��H = ��444.3 kJ��mol��1����S = ��280.1 J��mol��1��K��1
D.NH4HCO3(s) + CH3COOH(aq) = CH3COONH4 (aq) + CO2(g) +H2O(l)
��H = 37.30 kJ��mol��1����S = 184.0 J��mol��1��K��1
 ���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д�
���Ͱ�ͨ�������Сѧ��ʱͬ�����ϵ�д� ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.2KClO3(s)![]() 2KCl(s)+3O2(g)?
2KCl(s)+3O2(g)?
��H=-78.03 kJ��mol-1����S=1 110 J��mol-1��K-1?
B.CO(g)![]() C(s,ʯī)+
C(s,ʯī)+![]() O2��g��?
O2��g��?
��H=110.5 kJ��mol-1����S=-89.36 J��mol-1��K-1?
C.4Fe(OH)2(s)+2H2O(l)+O2(g)![]() 4Fe(OH)3(s)?
4Fe(OH)3(s)?
��H=-444.3 kJ��mol-1����S=-280.1 J��mol-1��K-1?
D.NH4HCO3(s)+CH3COOH(aq)![]() CO2(g)+CH3COONH4(aq)+H2O(l)?
CO2(g)+CH3COONH4(aq)+H2O(l)?
��H=37.301 kJ��mol-1����S=184.0 J��mol-1��K-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�У�һ�������Է����е��ǣ�������
��������KClO3(s)��2KCl(s) ����2(g)
���ȣ���78.03 kJ��mol��1 ���ӣ�1110 J��mol��1��K��1
�£�CO(g)��C(sʯī) ��1/2��2(g)��
���ȣ�110.05 kJ��mol��1 ���ӣ���89.36 J��mol��1��K ��1
���Fe(OH)2(s)��2H2O(l)��O2(g)����Fe(OH)3(s)
���ȣ���444.3 kJ��mol��1 ���ӣ���280.3 J��mol��1 ��K��1
�ģ�NH4HCO3(s)��CH3COOH(aq)��CO2(g)��CH3COO NH4(aq)��H2O(l)
���ȣ�37.30 kJ��mol��1 ���ӣ�184.05 J��mol��1 ��K��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�������и߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪����ϵ�������ܱ仯��G=��H-T��S<0ʱ����Ӧ���Է����С����з�Ӧ�У�һ�������Է����е���(�� ��)
A��2KClO3(s)=2KCl(s)+3O2(g)�� ��H=-78.03kJ/mol����S="1110" J/(mol��K)
B��CO(g)=C(s��ʯī)+1/2O2(g)�� ��H=110.5kJ/mol����S="-89.36" J/(mol��K)
C��4Fe(OH)2(s)+2H2O(l)+O2(g)=4Fe(OH)3(s)����H=-444.3kJ/mol��S="-280.1" J/(mol��K)
D��NH4HCO3(s)+CH3COOH(aq)=CO2(g)+CH3COONH4(aq)+H2O(l)
��H=37.301kJ/mol�� ��S="184.05" J/(mol��K)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com