
| ||
| ||


 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д� ����ϰ�⽭��ϵ�д�
����ϰ�⽭��ϵ�д� ѧ���쳵��������������������ϵ�д�
ѧ���쳵��������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧ����Ũ�������ʵ�ʵ�飺
ijѧ����Ũ�������ʵ�ʵ�飺
| ||
| ||
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
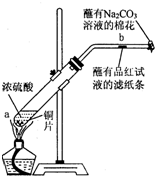
 ��һ֧�Թ��з���һ���С��ͭƬ���ټ�������Ũ���ᣬȻ����Թ̶ܹ�������̨�ϣ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������У������Թܿڣ��ڲ������ܿڴ�����һ��պ��Na2CO3��Һ���������Թܼ��ȣ��۲������Թ��е�Һ������ʱ��ֹͣ���ȣ��ش��������⣺
��һ֧�Թ��з���һ���С��ͭƬ���ټ�������Ũ���ᣬȻ����Թ̶ܹ�������̨�ϣ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������У������Թܿڣ��ڲ������ܿڴ�����һ��պ��Na2CO3��Һ���������Թܼ��ȣ��۲������Թ��е�Һ������ʱ��ֹͣ���ȣ��ش��������⣺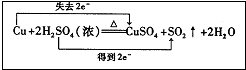

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ֧�Թ��з���һ���С��ͭƬ���ټ�������Ũ���ᣬȻ����Թ̶ܹ�������̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ������ܿڴ�����һ��պ����Һ���������Թܼ��ȣ��۲�������Ӧһ��ʱ���Ժ�ֹͣ���ȡ��ش��������⣺
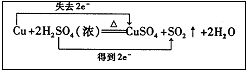
��1��д��a����Ӧ�Ļ�ѧ����ʽ ��
Ũ���������ֵ������� �ԡ�
��2���Թ��е�Һ�巴Ӧһ��ʱ�����ֽ���ı仯Ϊ �����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��3�����Թ���Һ����ȴ��ȡ�Թ��ϲ�Һ�����һ֧�Թ��У���������������ˮ���ɹ۲���Һ�� ɫ��
��4���������ܿڴ�����һ��պ��Na2CO3��Һ���������������
��5�����Ũ�����Ũ��Ϊ��ͭƬ�ǹ����ģ�����ʹ֮��Ӧ��������ԭ������Ϊ
����Ũ�����ʵ����� ����д�����ڡ��������ڡ���С�ڡ���
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�갲��ʡ���ض��и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ������
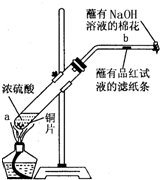
��һ֧�Թ��з���һ���С��ͭƬ���ټ�������Ũ���ᣬȻ����Թ̶ܹ���
����̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ������ܿڴ�����һ��պ��Na2CO3��Һ���������Թܼ��ȣ��۲�����Ӧһ��ʱ���ֹͣ���ȡ��ش��������⣺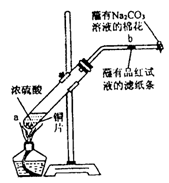
��1��д��a����Ӧ�Ļ�ѧ����ʽ ��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ �����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��3�����Թ��е�Һ����ȴ���Թ��ϲ�Һ�嵹ȥ���ٽ�ʣ��������������ˮ�У��ɹ۲���Һ�� ɫ��
��4���������ܿڴ�����һ��պ��Na2CO3��Һ��������������� ����Ӧ
�Ļ�ѧ����ʽΪ ��
��5������Ũ��Ϊ18 mol/L��Ũ����100 mL�������ͭƬ������ʹ֮��Ӧ����ԭ�����ᣨѡ����ڡ��������ڡ���С�ڡ��� 0.9 mol ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���㽭ʡ��ɽ���е���У��һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ������
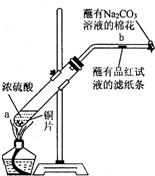
��12�֣�ijѧ����Ũ�������ʵ�ʵ�飺��һ֧�Թ��з���һ���С��ͭƬ���ټ���2mLŨ���ᣬȻ����Թ̶ܹ�������̨�ϡ���һС��պ��Ʒ����Һ����ֽ������е�����Ƥ���IJ������С������Թܿڣ��ڲ����ܿڴ�����һ��պ��Na2CO3��Һ�����������Թܣ��۲�����.�ش��������⣺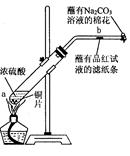
��1��д���Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��2���Թ��е�Һ�巴Ӧһ��ʱ���b����ֽ���ı仯Ϊ �����Թ��з�Ӧֹͣ�������ܷ���պ��Ʒ����Һ����ֽ�����ȣ���ֽ���ı仯Ϊ ��
��3��պ��Na2CO3��Һ������������ ��
��4��������������γɹ��̿������з�Ӧ�е� ����ʾ��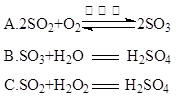
��5��Ũ������������Ҫ���ʣ����뺬��ˮ�ֵ��������ù����в�����ʾ��������
| A������ | B����ˮ�� | C��ǿ������ | D����ˮ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com