
 ����Ϊ��2-��-2��4-����ϩ��
����Ϊ��2-��-2��4-����ϩ�� ��һ��-Br����A�Ľṹ��ʽΪ��CH3��2CHCHBrCH3
��һ��-Br����A�Ľṹ��ʽΪ��CH3��2CHCHBrCH3
���� ��1���ٸ�������������ԭ��̼�����ij�飬����֧���Ѻű࣬����ǰͬ�ಢ�����Ӧ��һ���ߣ���
�� ����Һ���̼̼˫����̼������6��̼ԭ�ӣ�����̼̼˫���ͼ������̼ԭ��Ϊ1��̼ԭ�ӣ���2�š�4��̼ԭ���Ϻ���̼̼˫����Ϊ��ϩ����
����Һ���̼̼˫����̼������6��̼ԭ�ӣ�����̼̼˫���ͼ������̼ԭ��Ϊ1��̼ԭ�ӣ���2�š�4��̼ԭ���Ϻ���̼̼˫����Ϊ��ϩ����
��ijȲ����H2��ȫ�ӳɺ�IJ���ΪCH3CH2CH��CH3��C��CH3��3������̼ԭ����ÿ��̼ԭ��ȥ��2��Hԭ���������ʾ��Ǹ�Ȳ����
�ܸ���̼ԭ�ӳɼ���ʽȷ������ʽ�����������м�����ԭ�ӣ�����һ�ȴ����ͬ���칹����м��֣�
������-CH3��-Br��ֻ��һ���������ֻ�����ڴ��л������Χ��������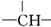 �ڴ��л���ṹ���ڲ���
�ڴ��л���ṹ���ڲ���
��2��ij�л���A����Է�������Ϊ74���Һ������ͼ��ͼ2��A�к��жԳƵļ����ԳƵ��Ǽ����Ѽ���
��
��3��1���ij����������ȫȼ�����ɵ�CO2�����ɵ�ˮ������1�������ͬ��ͬѹ�²ⶨ����˵��Hԭ�Ӹ�����Cԭ�Ӹ�����2����ʯ�����ն�����̼��ˮ�������ķ���ʽΪCxH2x+2��0.1mol������ȫȼ������0.1xmolCO2����x+1����0.1molˮ��������̼��ˮ������=0.1xmol��44g/mol+��x+1����0.1mol��18g/mol=39g��x=6��
�ݴ��жϸ����ķ���ʽ����������һ�ȴ�����3�֣�˵�����������к���3����ԭ�ӣ��ݴ��жϽṹ��ʽ��
��� �⣺��1����CH3CH��CH3��CH��CH2CH3��C��CH3��3����Һ���֧������̼������5��̼ԭ�ӣ�����ȡ�������������̼ԭ��Ϊ1��̼ԭ�ӣ����Ը�����������2��2��4-����-3-�һ����飬�ʴ�Ϊ��2��2��4-����-3-�һ����飻
�� ����Һ���̼̼˫����̼������6��̼ԭ�ӣ�����̼̼˫���ͼ������̼ԭ��Ϊ1��̼ԭ�ӣ���2�š�4��̼ԭ���Ϻ���̼̼˫����������������2-��-2��4-����ϩ���ʴ�Ϊ��2-��-2��4-����ϩ��
����Һ���̼̼˫����̼������6��̼ԭ�ӣ�����̼̼˫���ͼ������̼ԭ��Ϊ1��̼ԭ�ӣ���2�š�4��̼ԭ���Ϻ���̼̼˫����������������2-��-2��4-����ϩ���ʴ�Ϊ��2-��-2��4-����ϩ��
��ijȲ����H2��ȫ�ӳɺ�IJ���ΪCH3CH2CH��CH3��C��CH3��3������̼ԭ����ÿ��̼ԭ��ȥ��2��Hԭ���������ʾ��Ǹ�Ȳ�������Ȳ���ṹ��ʽΪHC��CCH��CH3��C��CH3��3������Ϊ3��4��4-����-1-��Ȳ��
�ʴ�Ϊ��HC��CCH��CH3��C��CH3��3��3��4��4-����-1-��Ȳ��
�ܸ���̼ԭ�ӳɼ���ʽȷ������ʽΪC9H8���÷����к���4����ԭ�ӣ�������һ�ȴ����ͬ���칹�����ĿΪ4��
�ʴ�Ϊ��C9H8��4��
������-CH3��-Br��ֻ��һ���������ֻ�����ڴ��л������Χ��������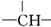 �ڴ��л���ṹ���ڲ��ұ˴����������Ӻ�õ��ṹ��
�ڴ��л���ṹ���ڲ��ұ˴����������Ӻ�õ��ṹ��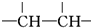 �����õĴ˽ṹ�����������ȫ�ȼۣ��ʽ�һ��-Br������-CH3��
�����õĴ˽ṹ�����������ȫ�ȼۣ��ʽ�һ��-Br������-CH3��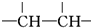 ���������õĽṹֻ��һ�֣�������CH3��2CHCHBrCH3��������A�Ľṹ��ʽΪ��CH3��2CHCHBrCH3���ʴ�Ϊ����CH3��2CHCHBrCH3��
���������õĽṹֻ��һ�֣�������CH3��2CHCHBrCH3��������A�Ľṹ��ʽΪ��CH3��2CHCHBrCH3���ʴ�Ϊ����CH3��2CHCHBrCH3��
��2������ͼ��֪���л�������ԭ������Ϊ74���������ͼ��ʾ���ڶԳƵļ����ԳƵ��Ǽ����Ѽ��ɵ÷��ӵĽṹ��ʽΪ��CH3CH2OCH2CH3��
�ʴ�Ϊ��CH3CH2OCH2CH3��
��3��1���ij����������ȫȼ�����ɵ�CO2�����ɵ�ˮ������1�������ͬ��ͬѹ�²ⶨ����˵��Hԭ�Ӹ�����Cԭ�Ӹ�����2����ʯ�����ն�����̼��ˮ�������ķ���ʽΪCxH2x+2��0.1mol������ȫȼ������0.1xmolCO2����x+1����0.1molˮ��������̼��ˮ������=0.1xmol��44g/mol+��x+1����0.1mol��18g/mol=39g��x=6��
�ݴ��жϸ����ķ���ʽC6H14����������һ�ȴ�����3�֣�˵�����������к���3����ԭ�ӣ�����ṹ��ʽΪCH3CH2C��CH3��3��
�ʴ�Ϊ��C6H14��CH3CH2C��CH3��3��
���� ���⿼����ۺϣ��漰�л������ʽ��ȷ�����л���������ͬ���칹�������жϵ�֪ʶ�㣬���ؿ���ѧ������������������ȷ�л���ṹ�ص㡢��ȼ�ն��ɡ�����ԭ���ǽⱾ��ؼ���ע�⣺ϩ����������һ������̼ԭ�Ӹ�����࣬��������һ�������й����ŵ�̼ԭ�ӣ���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2 C03��Һ�ʼ��ԣ�C032-+2H20?H2C03+20H- | |
| B�� | NaHC03��Һ�мӹ���Ca��OH��2��Һ��Ca2++20H-+2HC03-�TCaC03++C032-+2H2O | |
| C�� | Ư����Һ��ͨ�����������������壺ClO-+SO2+H2O�THC1O+HSO3- | |
| D�� | ��NaAl02��Һ��ͨ�����C02��AlO2-+CO2+2H2O�TAl��OH��3+HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Ũ������ʹ�������ۻ������ڳ�������������������Ũ���� | |
| B�� | ���ڴ�����ȼ�ջ�����º�ˮ������Ӧ���ܵõ�Fe3O4 | |
| C�� | ���������ڽ������˳�������Ԫ�ص�ǰ�棬����ǿ�ᷴӦһ���ų����� | |
| D�� | �����ۼ���FeCl3��CuCl2�����Һ�У���ַ�Ӧ��ʣ��Ĺ����в�һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| B�� | �ô����ȥˮ���е�̼��ƣ�CaCO3+2H+�TCa2++H2O+CO2�� | |
| C�� | ����������Һ������������Һǡ�÷�Ӧ�����ԣ�2H++SO42-+Ba2++2OH-�T2H2O+BaSO4�� | |
| D�� | ��ⱥ��MgCl2��Һ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����ȣ�SO2Cl2����һ����Ҫ�Ļ����Լ���ʵ���Һϳ������ȵ�ʵ��װ����ͼ��ʾ��
�����ȣ�SO2Cl2����һ����Ҫ�Ļ����Լ���ʵ���Һϳ������ȵ�ʵ��װ����ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.25 | B�� | 0.1 | C�� | 0.5 | D�� | 0.05 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com