
����Ŀ������˵����ȷ����( )
A. ��ϵͳ�����������л���![]() ��
�� ������̼ԭ������Ϊ7��
������̼ԭ������Ϊ7��
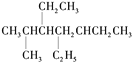
B. ij���ķ���ʽΪC10H14��������ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ���ҷ��ӽṹ��ֻ��һ���������������������4��
C. ̼ԭ����С�ڻ����8�ĵ�ϩ���У���HBr�ӳɲ���ֻ��һ�ֽṹ�����������ĵ�ϩ����6��
D. ���ⶨC3H7OH��C6H12��ɵĻ������������������Ϊ8%����˻������̼������������78%
���𰸡�D
�����������������A��![]() ��������8��̼ԭ�ӣ���A���� B�������ķ���ʽ����CnH2n-6��ͨʽ��������ʹ��ˮ��ɫ������ʹKMnO4������Һ��ɫ�����Ժ��б�������������֪���÷��Ӻ��ж������������ֻ�ж϶��������ͬ���칹�弴�ɣ����������̼��ͬ���칹����-��CH2��2CH3��-CH2CH��CH3��2��-CH��CH3��CH2CH3��-C��CH3��3������ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����-C��CH3��3�뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����������������3�֣���B����C�����������ĵ�ϩ����4�֣�̼ԭ����С��8�ĵ�ϩ����HBr�����ӳɷ�Ӧֻ��һ�ֲ��˵���õ�ϩ������̼̼˫��Ϊ���ĵĶԳƽṹ��������һ�������У�CH2�TCH2��CH3-CH�TCH-CH3��CH3-CH2-CH�TCH-CH2-CH3����CH3��2C=C��CH3��2����C����D��C3H7OH��O����������Ϊ
��������8��̼ԭ�ӣ���A���� B�������ķ���ʽ����CnH2n-6��ͨʽ��������ʹ��ˮ��ɫ������ʹKMnO4������Һ��ɫ�����Ժ��б�������������֪���÷��Ӻ��ж������������ֻ�ж϶��������ͬ���칹�弴�ɣ����������̼��ͬ���칹����-��CH2��2CH3��-CH2CH��CH3��2��-CH��CH3��CH2CH3��-C��CH3��3������ͬϵ�����뱽��������Cԭ���ϱ��뺬��Hԭ�ӣ��ſɱ����Ը������������ʹ���Ը��������Һ��ɫ�����-C��CH3��3�뱽��������Cԭ���ϲ���Hԭ�ӣ�����ʹ���Ը��������Һ��ɫ����������������3�֣���B����C�����������ĵ�ϩ����4�֣�̼ԭ����С��8�ĵ�ϩ����HBr�����ӳɷ�Ӧֻ��һ�ֲ��˵���õ�ϩ������̼̼˫��Ϊ���ĵĶԳƽṹ��������һ�������У�CH2�TCH2��CH3-CH�TCH-CH3��CH3-CH2-CH�TCH-CH2-CH3����CH3��2C=C��CH3��2����C����D��C3H7OH��O����������Ϊ![]() �����Ի������C3H7OH��������Ϊ
�����Ի������C3H7OH��������Ϊ![]() =30%�����Ի������C6H12����������Ϊ1-30%=70%���������C3H7OH��HԪ���ڻ�����е���������Ϊ
=30%�����Ի������C6H12����������Ϊ1-30%=70%���������C3H7OH��HԪ���ڻ�����е���������Ϊ![]() ��30%=4%���������C6H12��HԪ���ڻ�����е���������Ϊ
��30%=4%���������C6H12��HԪ���ڻ�����е���������Ϊ![]() ��70%=10%�����Ի�����������������ΪH%=4%+10%=14%���������C����������Ϊ1-14%-8%=78%����D��ȷ����ѡD��
��70%=10%�����Ի�����������������ΪH%=4%+10%=14%���������C����������Ϊ1-14%-8%=78%����D��ȷ����ѡD��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ɶ�����Ԫ����ɵ���ѧ�����ĺ���Ԫ�ص�����A��B��C��D,������ͼת����ϵ(����������ͷ�Ӧ��������ȥ)��

��1����AΪNa ,��EΪ________, A��ˮ��Ӧ�����ӷ���ʽΪ____________________
��2����AΪNa2O2 ,��EΪ________, A��CO2��Ӧ�Ļ�ѧ����ʽΪ____________________,ÿ��1mol Na2O2�μӷ�Ӧ��ת�Ƶ�����Ϊ________NA
��3����A������Na����Na2O2,����ת����ϵ�ж�����B��________����C��________
���͵�C��Һ��ͨ��CO2��������ɫ���壬�þ���Ϊ________���û�ѧ����ʽ��ʾ�䷴Ӧԭ��Ϊ��_____________________
����1mol/L��B��Һ��μ��뵽1L1mol/L��AlCl3��Һ�У�������ɫ����39g�����������B��Һ���������Ϊ________L����________L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ǽ������ϵ����ǣ���Ԫ�صĵ��ʼ��仯�����ڻ�������Դ�����Ͽ�ѧ��������ҪӰ��㷺Ӧ�á��Իش��������⣺
(1)������Ȼ��������Ҫ�Ĵ�����ʽ��_______��_______��
(2)������������豸����ˮ����Ƭ �ڼ����оƬ ��������� �ܹ�̫���ܵ�� ��ʯӢ���ά�����������д���õ��赥�ʵ���_________�����ò�����ҪΪSiO2����_________��
(3)д����ҵ����ȡ�ֹ�Ļ�ѧ��Ӧ����ʽ___________�����У�������Ϊ_____________����ԭ��Ϊ_______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����Ȼ�ѧ����ʽ��2H2��g����O2��g��===2H2O��g������H=��483��6 kJ/mol
H2��g����![]() O2��g��===H2O��g������H=��241��8 kJ/mol
O2��g��===H2O��g������H=��241��8 kJ/mol
H2��g����![]() O2��g��===H2O��l������H=��285��8 kJ/mol
O2��g��===H2O��l������H=��285��8 kJ/mol
��������ȼ����Ϊ
A. 438��6 kJ/mol B. 241��8 kJ/mol C. 285��8 kJ/mol D. ��ȷ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijKCl��Ʒ�к�������K2CO3��K2SO4�Ͳ�����ˮ�����ʡ�Ϊ���ᴿKCl���Ƚ���Ʒ��������ˮ�У����衢���ˣ��ٽ���Һ����ͼ��ʾ��������ᴿ(���˲�������ȥ)������˵����ȷ����

A����ʼ��Һ������pH��7 B���Լ���ΪBa(NO3)2��Һ
C����ͼ�����뾭2�ι��� D��������Ŀ���dz�ȥCO32��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
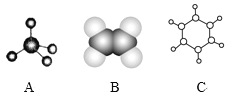
����Ŀ����ͼ��A��B��C�ֱ��������л���Ľṹģ�ͣ�
��ش��������⣺
��1��A��B����ģ�ͷֱ����л���____ģ�ͺ�___ģ�͡�
��2��A����ͬϵ��ķ���ʽ����ͨʽ____(��n��ʾ)����n��____ʱ��������ʼ����ͬ���칹�塣
��3��A��B��C�����л����У�����ԭ�Ӿ��������___(������)���ṹ��ʽΪ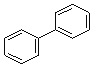 ���л����У�����ͬһƽ���ڵ�̼ԭ��������Ϊ____��
���л����У�����ͬһƽ���ڵ�̼ԭ��������Ϊ____��
��4���л���C�����еĽṹ��������_____(����ĸ���)��
a����̼̼˫����̼̼��������Ľṹ b���ж���������ˮ���ܶȱ�ˮС
c������ʹ����KMnO4��Һ����ˮ��ɫ d��һ������������������������Ӧ
��5���������������л�����ȫȼ������H2O��CO2���������������(��ͬ״����)������__(��A��B��C)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڶ����������������ȷ����
A.�������辧������SiO2���ӹ��ɵģ������۵�ߣ�Ӳ�ȴ�
B.������ʯӢ���������ռ�
C.�����������������ʯӢ�ӱ���ѹ����Ϻ��ά
D.�������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��(C6H10O5)n![]() C6H12O6
C6H12O6![]() CH2OH(CHOH)4COOH�� ����˵����ȷ����( )
CH2OH(CHOH)4COOH�� ����˵����ȷ����( )
A. (C6H10O5)n��C6H12O6��Ϊͬϵ��
B. ��Ӧ�����ڼӳɷ�Ӧ
C. ��ٷ�Ӧ�����Һ��ֱ�ӵμ�������Һ�����ȣ�����֤C6H12O6������
D. 1 mol CH2OH(CHOH)4COOH�������6 molNa
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com