
25��ʱ�������и���Һ��������ȷ����
A��pH��2��������pH��l��������Һ��c��H����֮��Ϊ2 ��1
B��4��pH��ͬ����Һ����CH3COONa ��C6H5ONa ��NaHCO3 ��NaOH������Һ�����ʵ���Ũ���ɴ�С��˳���Ǣ�>��>��>��
C��0��1 mol/LHA��ij�ᣩpH��3��0��1 mol/L BOH��ij�pH��13����BA���Σ�����ҺpH<7
D��pH��3�Ĵ�����Һ��pH��11��NaOH��Һ�������ϣ�c��Na��>c��CH3COO��>c��H��>c��OH�� 2010��֣�ݸ��б�ҵ�����������Ԥ�����ۻ�ѧ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| V��HCl��/mL | 0.00 | 12.00 | 18.00 | 22.00 | 23.00 | 23.96 | 24.00 | 24.04 | 25.00 | 26.00 | 30.00 |
| pH | 13.1 | 12.6 | 12.2 | 11.7 | 11.4 | 9.9 | 7.0 | 4.0 | 2.7 | 2.4 | 1.9 |
| ָʾ�� | ��ɫ��Χ ��pH�� |
����Χ����ɫ | ||
| ǰ | �м� | �� | ||
| ���� | 3.1��4.4 | �� | ��ɫ | �� |
| ʯ�� | 5.0��8.0 | �� | ��ɫ | �� |
| ��̪ | 8.2��10.0 | �� | �ۺ� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ��ϵ pH[ |
��ͬ�¶���NaBH4�İ�˥�ڣ�min�� | |||
| 0�� | 25�� | 50�� | 75�� | |
| 8 | 4.32��100 | 6.19��10-1 | 8.64��10-2 | 1.22��10-2 |
| 10 | 4.32��102 | 6.19��101 | 8.64��100 | 1.22��100 |
| 12 | 4.32��104 | 6.19��103 | 8.64��102 | 1.22��102 |
| 14 | 4.32��106 | 6.19��105 | 8.64��104 | 1.22��104 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��������Ͽ��������ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��ϩ�����ںϳ�ɱ�������߳��ũҩ������ʽΪC3H5Br2Cl����Ӧ�ù㷺��DAP��֬��
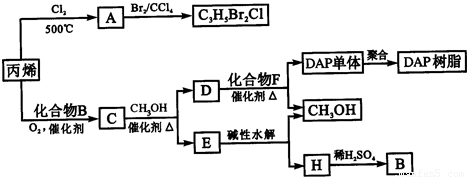
��֪���봼�ɷ���������������Ӧ��
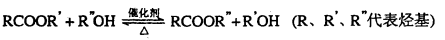
��1��ũҩC3H5Br2Cl������ÿ��̼ԭ���Ͼ�����±ԭ�ӡ�
A�Ľṹ��ʽ��__________________��A�����������ŵ�������____________________��
��ϩ��A�ķ�Ӧ������_______________��A��C3H5Br2CI�ķ�Ӧ������_____________��
��2��Aˮ��ɵõ�D����ˮ�ⷴӦ�Ļ�ѧ����ʽΪ��
_______________________________________________________________________.
��3��C�����ܶ�����ͬ״̬�¼����ܶȵ�6.25����S�и�Ԫ�ص����������ֱ�Ϊ��̼60%����8%����32%. S�Ľṹ��ʽΪ_________________________________��
��4������˵����ȷ����______________������ĸ���ţ���
a��C�ܷ����ۺϷ�Ӧ����ԭ��Ӧ��������Ӧ
b��C����2������������ͬ���칹����4��
c��D������IJ�����B������ͬ����Է�������
d��E���з�����ζ���������Ҵ�
��5��E��ˮ����ᆳ�������յõ��״���B�����߾���ѭ��������DAP��֬���Ʊ������н��״���H����IJ���������__________________��
��6��F�ķ���ʽΪC10H10O4. ��DAP����Ϊ���Ķ�Ԫȡ���������ȡ���������ڶ�λ���õ��屽���ϵ�һ��ȡ����ֻ�����֡�����D��F��Ӧ����DAP����Ļ�ѧ����ʽΪ��
_______________________________________________________________________.
��7��ʵ������2-�����Ʊ���ϩʱ������������SO2�� CO2 ��ˮ������ijͬѧ�������Լ�
�������������壬�������ͨ���Լ���˳����_______________������ţ���
�ٱ���Na2SO3��Һ������KMnO4��Һ ��ʯ��ˮ ����ˮCuSO4��Ʒ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
ijѧϰ��ȤС��̽���ϸɵ�أ�пͲ������̿�ۡ�MnO2��NH4Cl�ȵĺ�״��Ļ������ã����û��յ����ʽ�������ʵ�顣
I����1���ӷϸɵ������ȡNH4Cl��
�� ���øú�״����ȡNH4Clǰ�����IJ���Ϊ��a���ܽ� b�� ��
�� ��ͬѧ���룺���������NH4Cl��Һ�������ᾧ�����գ��Ϳ����Ƶô�����NH4Cl����Լ�ͬѧ�ķ����������۲�˵�����ɣ�______________________________________
��
��2����ȡ������
�� ��ͬѧҪ�Ʊ����ռ��������İ��������и���Ӧ�������к�������
a�����Ȼ�粒�����ȷֽ� b����Ũ��ˮ�����������ƹ�����
c�����������ƹ������Ũ��ˮ�� d�����Ȼ��Ũ��Һ�����������ƹ�����
�� ��ͬѧ��Ϊ������ƿ���������ϣ���ͼ��ʾ�����Ϳ����ռ��������İ�����
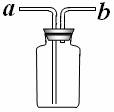
������Ϊ���У���˵���������_____________________________��
������Ϊ�����У���˵�������ɣ�___________________________��
��ͬѧ��������õ��İ�����ȡ��ˮ������������̽��ʵ�飺
��3��Ϊ̽��NH3?H2O�Ƿ���������ʡ�
��ͬѧ��Ʒ������£��� ��1.12L�������NH3��ȫ����ˮ�������Һ500mL��
�� ���۷������ݣ���
�ɵó����ۡ�
������ʵ�鲽��ڣ���д������Ŀո��С�
��4��̽����ˮ������ķ�Ӧ���̡�
��ͬѧ����������ʵ�飺��25mL������ˮ����εμ�ͬŨ�ȵ����ᣬ�ⶨ��Ӧ��������ҺpH��������pH�仯���ߣ���ͼ������ش�
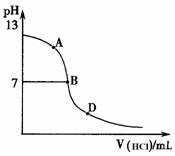
�����ǡ����ȫ�к�ʱ��pH��Ӧ��_________
����A��B��D������ʱ��Һ��c(NH3?H2O)��c(NH4+)��______mol?L��1����Һ��
�����ӵ�Ũ���ɴ�С��˳��Ϊ ��
III�������̽��
��5�����������̽��ʵ�鱨�档
��̽�����⡿�Ƚ���25�桢0.1mol?L-1��NH3?H2O��Һ��0.1mol?L-1��NH4Cl��Һ�У�NH3?H2O�ĵ���̶���NH4+ˮ��̶ȵ���Դ�С��
��̽��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
��08�Ͳ���ģ�⣩��18�֣�ijѧϰ��ȤС��̽���ϸɵ�أ�пͲ������̿�ۡ�MnO2��NH4Cl�ȵĺ�״��Ļ������ã����û��յ����ʽ�������ʵ�顣
I����1���ӷϸɵ������ȡNH4Cl��
�� ���øú�״����ȡNH4Clǰ�����IJ���Ϊ��a���ܽ� b�� ��
�� ��ͬѧ���룺���������NH4Cl��Һ�������ᾧ�����գ��Ϳ����Ƶô�����NH4Cl����Լ�ͬѧ�ķ����������۲�˵�����ɣ�______________________________________
��
��2����ȡ������
�� ��ͬѧҪ�Ʊ����ռ��������İ��������и���Ӧ�������к�������
a�����Ȼ�粒�����ȷֽ� b����Ũ��ˮ�����������ƹ�����
c�����������ƹ������Ũ��ˮ�� d�����Ȼ��Ũ��Һ�����������ƹ�����

�� ��ͬѧ��Ϊ������ƿ���������ϣ���ͼ��ʾ�����Ϳ����ռ��������İ�����
������Ϊ���У���˵���������_____________________________��
������Ϊ�����У���˵�������ɣ�___________________________��
��ͬѧ��������õ��İ�����ȡ��ˮ������������̽��ʵ�飺
��3��Ϊ̽��NH3?H2O�Ƿ���������ʡ�
����ͬѧ��Ʒ������£��� ��1.12L�������NH3��ȫ����ˮ�������Һ500mL��
�� ���۷������ݣ���
�ɵó����ۡ�
������ʵ�鲽��ڣ���д������Ŀո��С�
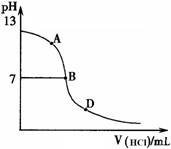 ��4��̽����ˮ������ķ�Ӧ���̡�
��4��̽����ˮ������ķ�Ӧ���̡�
��ͬѧ����������ʵ�飺��25mL������ˮ����
�εμ�ͬŨ�ȵ����ᣬ�ⶨ��Ӧ��������ҺpH����
����pH�仯���ߣ���ͼ������ش�
�����ǡ����ȫ�к�ʱ��pH��Ӧ��_________
����A��B��D������ʱ��Һ��c(NH3?H2O)��c(NH4+)��______mol?L��1����Һ��
�����ӵ�Ũ���ɴ�С��˳��Ϊ ��
III�������̽��
��5�����������̽��ʵ�鱨�档
��̽�����⡿�Ƚ���25�桢0.1mol?L-1��NH3?H2O��Һ��0.1mol?L-1��NH4Cl��Һ�У�NH3?H2O�ĵ���̶���NH4+ˮ��̶ȵ���Դ�С��
��̽���������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com